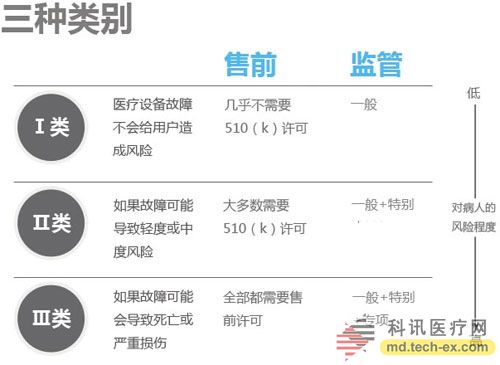
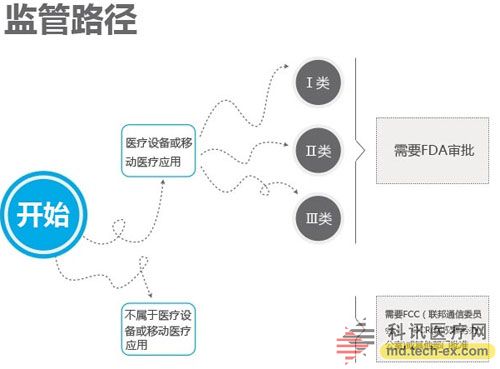
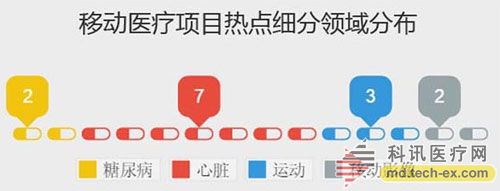
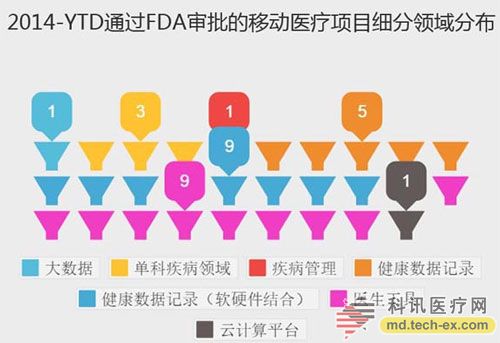
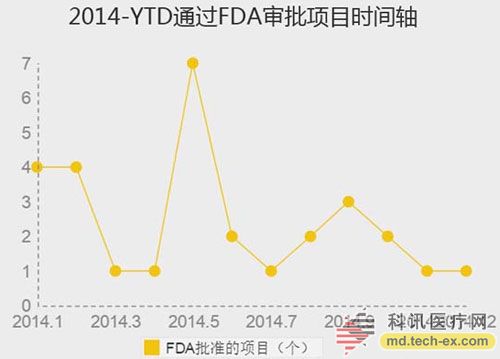
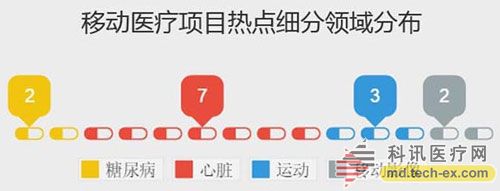
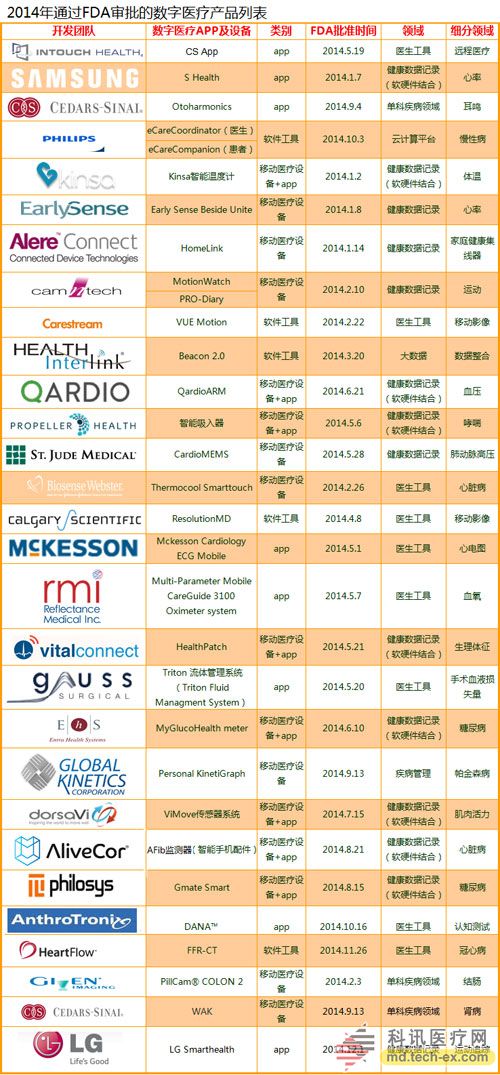
Release date: 2014-12-09 In order to avoid the potential risks of mobile medical applications lack of supervision, the US Food and Drug Administration (FDA) also officially announced the final end of the Mobile Medical Application Final Guidance in September 2013. Version, mobile medical development has also begun to have preliminary compliance standards, which is more conducive to the development of mobile medical. Like the medical devices regulated by the MDDS (Medical Device Data System), the FDA's principles governing mobile medical applications are also divided into three categories, the division of which is the functionality of mobile medical applications that, if not functioning properly, causes patients The degree of security risk varies. Alive, your director of Cor, Michael Righter once said that the FDA essentially requires software supervision. Generally, good software engineers will do these things naturally, like code review, unit testing, system level testing, change monitoring, etc. Experienced developers are required to operate closely on software deployments. FDA Digital Health Product Approval Concise Guide According to the FDA, there are three types of mobile medical applications that need to be supervised. Class I medical devices are information that can link and control one or more medical devices, or display, store, analyze, and transmit specific medical devices, such as The remote display shows the data monitored by the patient bedside monitor, displays the brain wave map, controls the sphygmomanometer cuff inflation and deflation. Some of these types of applications may require only one 510(k) application, premarket notification before entering the market, and are simpler than other types of apps. Class II medical devices are functions that can be converted into regulated mobile medical devices by means of accessories, displays, sensors, or medical devices that are currently regulated by regulations, such as attaching mobile devices to blood glucose test strips. The App on the device is then used to check the blood glucose to make the mobile device a blood glucose meter. Almost all such medical devices are required to automatically submit a 510(k) application and make a pre-sales notice. Class III medical devices are stand-alone software that can perform patient-specific analysis, provide specific patient diagnostics or treatment recommendations, and analyze the medical device data, which the FDA considers to be an adjunct to medical devices. Therefore, the classification it supervises will be the same as the classification of the highest risk equipment in various materials. This type of medical device must be approved by the FDA for premarket approval. During this period, clinical trials are required, and the time required for different products varies, several years or even longer, and the cost is high. Personalized KinetiGraph, a mobile medical device developed by Global Kinetics, Inc., which monitors and treats Parkinson's disease, provides comprehensive automated reporting of Parkinson's activities, making it easier for neurologists and other physicians to identify motor symptoms. Change to help make decisions to optimize treatment. What is the difference between the three types of medical equipment? For example, in the case of a bandage, the ordinary bandage is only for covering the wound and has no other effect, and belongs to the class I; it is connected with the mobile phone, and can detect the temperature of the wound through application, and may also be infected with a wound, and there is a slight risk to the consultation, belonging to class II; After connecting the mobile phone, not only the wound related information can be displayed, but also the feedback and guidance of the patient can be given through the drug algorithm, and it is classified into the class III. Once you have identified your product, then combine the appropriate regulatory path and business model. If your model is a fee, then you may need to publish clinical results and obtain FDA approval. The following is a summary of the FDA's approval of the FDA's approval of mobile medical (smartphone-connected medical devices and stand-alone applications (Apps)). In 2014, the FDA has approved 31 digital health products to date. FDA approved mobile medical programs are concentrated in 4 categories The categories of mobile medical items approved by the FDA 510(k) this year mainly include four categories: app, software tools, mobile medical devices, and mobile medical device apps. Except for a small number of software tools, the other categories are comparable, and the software tools are subdivided. The field is mainly doctor tools - mobile imaging industry. The most FDA-approved projects in the field of doctor tools There are seven sub-areas in the mobile medical project data approved by the FDA from the Arterial Network Internet Medical Research Institute this year. Among them, there are 9 items related to health data records (software and hardware combination); 5 items that do not combine health data records of other medical products; 9 items of doctor tools; 3 items in the field of single diseases; the rest of the big data, Disease management and cloud computing platforms each account for one. 2014 so far, the time trend of FDA project approval, from the project field, there are only two areas in May, doctor tools and health data records, it seems that FDA on these two relatively low-risk areas, "gun Slightly raise one inch." FDA audit project hotspots In this year's approval project, the Arterial Network Internet Medical Research Institute counted the more focused uses, mainly diabetes, heart disease, motion tracking, and mobile imaging. The most involved projects involving the heart, including mobile medical devices that measure heart rate. Used as a doctor's tool for treatment and imaging diagnosis, for heart monitors used by patients. Which developers are interested in applying for FDA? The development of the FDA's developer of digital medical products in 2014 reflects the following trends: 1. The number of startups that have been established for less than 10 years is the largest, with 16 in total. This also shows that Internet medical startups are stepping up to break through regulatory barriers. 2. Some established medical equipment companies are making efforts to achieve breakthroughs and innovations. For example, eCare Companion, a cloud computing platform built by Philips, and an electrocardiogram monitoring app developed by McKesson. 3, some functions are closely related to medical serious medical treatment, the risk is very small, and the products that are more inclined to the fitness field are also applied for approval of the FDA. For example, just a few days ago, LG products mobile health application LGSmarthealth obtained FDA approval for tracking activities, calories, etc., in fact, does not require FDA permission, because these do not involve the purpose of diagnosis, treatment and prevention of any disease. Industry speculation, perhaps LG has plans to deepen the layout of the medical field for this product. 4. Compared with patients' DIY behavior, mobile medical tools used by doctors are often closely related to diagnosis and treatment, and their safety risk index is also high, which has become one of the key areas for applying for FDA. Attachment: Summary of 2014-YTD approved FDA-approved mobile medical projects Source: Arterial Network Internet Medical Research Institute Carbon Black, also known as carbon black, is an amorphous carbon. It is a light, loose and extremely fine black powder with a very large surface area, ranging from 10~3000m2/g. It is the product of carbonaceous materials (coal, natural gas, heavy oil, fuel oil, etc.) under the condition of insufficient air after incomplete combustion or thermal decomposition. Made by natural gas called "gas black", made by oil called "lamp black", made by acetylene called "acetylene black". In addition, there are "tank black", "furnace black". According to the carbon black performance is divided into "reinforced carbon black", "conductive carbon black", "wear-resistant carbon black", etc. Can be used as a black dye, used in the manufacture of Chinese ink, ink, paint, etc., also used as a rubber reinforcement. Imported Premium Carbon Black,Food Contact Black Carbon,Gas Channel Black,Premium Carbon Black Xingbang High Molecular Materials Co., Ltd. , https://www.chemicaladditive.com