Wanyuan Garlic Foods has founded in 2005.Which is a manufacturer and exporter of Vacuum fried,Fried and Dehydrated vegetables.We also got a patent for VF products.
The Color of Vaccum Fried Carrot is same as fresh Carrot.
Crispy VF carrot can be eat direcely ,and mixed with other VF vegetables.
Vacuum Fried Carrot products Huaiyang County Wanyuan Garlic Foods Processing Industries Co.,Ltd , https://www.wanyuangarlicfood.com
Waters Corporation (Milford, MA, USA)
Application Benefits â– Rapid separation of the isomers in the ruthenium complex enables real-time monitoring of material purification.
â– Simultaneous separation of the isomers and optical isomers in the ruthenium complex during a single chromatographic run to achieve an accurate assessment of purity, which requires multiple chromatographic separations in other systems.
â– Simple conversion from UPC 2TM to semi-preparative supercritical fluid chromatography (SFC) to purify target isomers and easily recover collected components under reduced conditions, reducing isomer formation. Thereby, a high purity material required for the manufacture of an organic light emitting diode (OLED) device is obtained.
Waters Solutions
ACQUITY UPC 2TM System
Investigator SFC System
EmpowerTM 3 software
ChromScopeTM software
ACQUITY UPC 2 BEH and BEH 2-EP Columns
Keywords <br>铱 complex, OLED, isomer, face, warp, enantiomer, convergent chromatography, UPC 2
INTRODUCTION <br>The synthesis and characterization of cyclic metal ruthenium(III) complexes in organic light-emitting diode (OLED) applications has attracted considerable interest because these complexes have high luminescence quantum yields and can be easily The synthetic method systematically modifies the ligand to adjust the color. Depending on the type of ligand surrounding the central ruthenium atom, these organometallic complexes may be classified as homogeneous and heteroligand. Isomers may be present in both the homo- and di-complexes, and these isomers are referred to as meridional (mer) and facial (fac) isomers. Isomers have different photophysical and chemical properties 1-3 that can affect the performance and longevity and stability of OLED devices. In addition, the heteroconjugates have optical isomerism. The enantiomer-rich complex emits a circular polarized light that can be used for three-dimensional electronic display 4 .
A variety of isomeric forms pose particular challenges for the purity assessment of these materials and the separation of isomers required to understand the failure mechanism of luminescent devices. This challenge is compounded by the current popular purification methods for these materials (ie, sublimation) 5-6 . Intramolecular thermodynamic isomerization may occur during sublimation. The purification process typically produces an isomeric mixture rather than the intended single isomer for equipment production, resulting in reduced performance. Obviously, the development of purification techniques under mild conditions is of great significance for reducing isomerization.
Since most of the cyclic metal ruthenium complexes have low solubility, the chromatographic analysis methods of the ring metal ruthenium complexes generally use normal phase liquid chromatography (NPLC). Supercritical fluid chromatography (SFC) and more advanced ultra-high performance convergent chromatography (UPC 2 ) provide an attractive alternative to normal phase chromatography, which increases resolution, reduces analysis time, and reduces organic solvent consumption. In this application note, we have three [2(2,4-difluorophenyl)pyridine]iridium(III)(Ir(Fppy) 3 ) and bis(4,6-difluorophenyl)pyridine C2,N The structure of Formyl ruthenium (III) (Flrpic) was separated using Waters ® ACQUITY UPC 2 , as shown in Figure 1. The feasibility of using SFC for the purification of Flrpic also illustrates the feasibility of using the Waters Investigator SFC system. 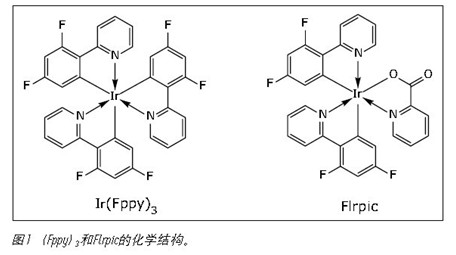
experiment
Instrumentation : All analytical experiments were performed on an ACQUITY UPC 2 controlled by Empower 3 software. Preparation experiments were performed on an Investigator SFC system controlled by ChromScope software.
Columns : Waters' ACQUITY UPC 2 BEH and 2-Ethyl Pyridine 3.0 x 100 mm, 1.7 μm columns. CHIRALPAK AS-H 4.6 x 150 mm, 5 μm, available from Chiral Tec hnologies (West Chester, PA).
Sample Description <br> Samples were purchased from Sigma Aldrich and 1-Material. In order to form an isomer, the sample is placed in a temperature control box for heat stress to initiate an isomerization reaction. After cooling to room temperature, the sample was dissolved in chloroform for subsequent analytical procedures.
Results and Discussion <br> Figure 2 is a UPC 2 /UV chromatogram of Ir(Fppy) 3 without treatment and heat stress. The peak 1 of the chromatogram is the same as the mass spectrum of the peak 2 (not shown), but the ultraviolet spectrum (inset) is significantly different, indicating that they are most likely to be a isomer and a warp. The reason for the peak marked "desfluoro" is that one of the F atoms in Ir(Fppy) 3 is lost. However, the main difference between the two spectra is the relative ratio between peak 1 and peak 2. When heated, the peak ratio of 1/2 will increase. It may be caused by a thermal isomerization process in which the less stable isomer (peak 2) is converted to a more stable isomer (peak 1). Figure 2 clearly shows that the isomer of Ir(Fppy) 3 can be easily separated by using ACQUITY UPC 2 . 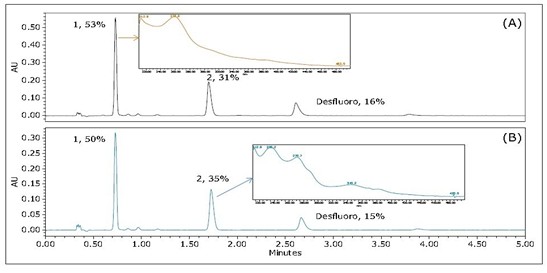
Figure 3 is a Flrpic UPC 2 /UV chromatogram obtained using an achiral stationary phase and a chiral stationary phase. In the chiral column, the Flrpic split is divided into two peaks, as shown in Figure 3B. The two peaks in Figure 3B have the same mass to charge ratio (not shown) and the ultraviolet spectrum (inset), indicating that these two peaks are most likely to be derived from the same pair of enantiomers. Unlike the homogeneous ligand Ir(Fppy) 3 , the heteroleptic Flrpic is composed of two different ligands. This molecular symmetry in turn produces optical isomerism. In practical applications, such as three-dimensional display, it is advantageous to have a high degree of illuminance asymmetry. Therefore, UPC 2 provides a simple method for determining the enantiomeric ratio of chiral fluorescent compounds, which is important for correlating chemical structures with luminescence symmetry. 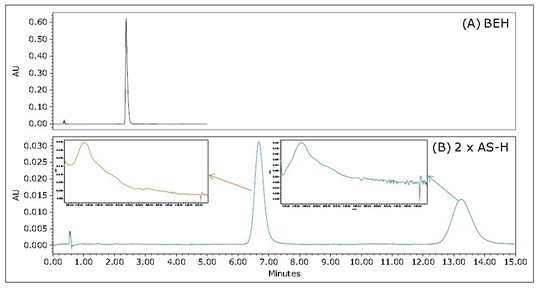
Figure 4 is an untreated and heat-stressed Flrpic UPC 2 /UV chromatogram obtained on an ACQUITY UPC 2 BEH column. For heat-stressed samples, an extra peak was observed, as shown in Figure 4B. The mass spectra of the two peaks were identical (results not shown). More closely observed in the UV spectrum (as shown in Figure 5), the UV spectra of the individual peaks in Figure 4B are not identical. Unlike the enantiomers shown in Figure 3B, the UV spectra of these enantiomers are identical. The small absorption peak λmax of the small peak in Fig. 4B is 245 nm, and the maximum absorption wavelength λmax of the main peak is 251 nm. These results indicate that the heat-stressed sample has been isomerized to form another isomer, similar to what was observed during sublimation 5,6 . Because the total analysis time is less than 5 minutes, UPC 2 can quickly determine the purity of materials after sublimation and can be used as a quality control method before equipment manufacturing. 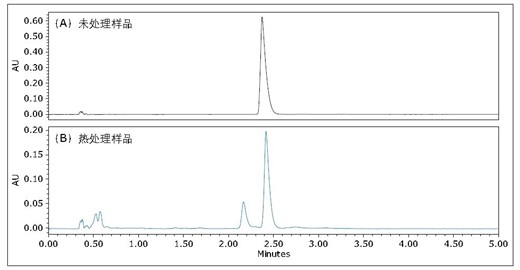
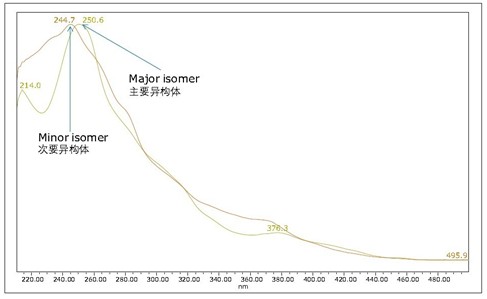
In theory, each isomer contains a pair of enantiomers. Therefore, we attempted to simultaneously isolate the four isomers of heat-stressed Flrpic, as shown in Figure 4B. The obtained ultraviolet spectrum is shown in Fig. 6. E1/E1' and E2/E2' are two pairs of enantiomers, while E1/E2 and E1'/E2' are two pairs of isomers. 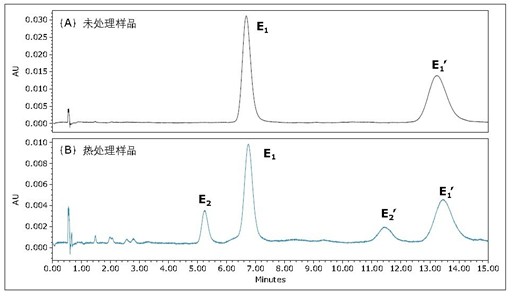
The isomer separation results in Figure 6 exceeded the results of the simple analysis. As a primary purification method for the cyclic metal ruthenium complex used in luminescent devices, sublimation causes unfavorable intramolecular thermal isomerization, as shown in Figures 2, 4, 6, and other figures 5-6 . Therefore, the use of isomer mixtures rather than pure substances in equipment often results in reduced performance and reduced life. The separation shown in Figure 6 illustrates that supercritical chromatography is expected to replace sublimation as a purification method for these materials.
Figure 7 is a SFC/UV chromatogram of heat-stressed Flrpic obtained using semi-preparative supercritical chromatography. Baseline resolution of all four isomers can be obtained. At 50 ° C, isopropanol was used as a co-solution, and the pure isomers were recovered under mild conditions, thereby reducing the possibility of isomer formation. It should be noted that although both FIG. 6B and FIG. 7 are obtained under the same chromatographic conditions, the resolution in FIG. 6B is much higher than that in FIG. The increase in resolution is largely due to the minimization of the volume of the UPC 2 system, which causes a decrease in peak dispersion. 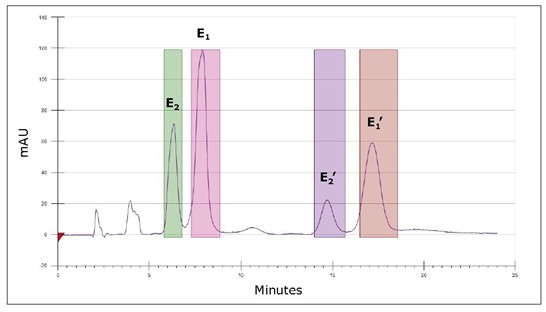
Figure 7. SFC/UV chromatogram of heat-stressed Flrpic obtained on a Waters Investigator SFC system using a CHIRALPAK AS-H 4.6 x 150 mm column (each 0.5 μm). The flow rate was 3 mL / min, the back pressure was 2175 p si, and the 23% isopropanol auxiliary solution was isocratically eluted; the temperature was 50 °C. The shaded area indicates the collected components.
Conclusions In this application, we discussed the separation of the iridium ligand Ir(Fppy) 3 and the Flrpic isomer by using ultra-high performance convergent chromatography. For Ir(Fppy) 3 , the face and warp isomers can be easily separated within 5 minutes. For Flrpic, the four isomers, whether isomeric or optically isomerized, are simultaneously separated in one separation operation.
The separation method proposed herein can increase the level of traditional analytical techniques used for purification evaluation. Purification evaluation is one of the analytical challenges in the synthesis, process and production of OLED devices and related materials. In addition, the supercritical fluid technology can also convert the UPC 2 method to the preparation method of the semi-preparative supercritical chromatography instrument to separate the target substance.
references
1. Kappaun S, Slugovc C, List EJW. Phosphorescent organic light-emitting devices: Working principle and iridium based emitter materials. Int J Mol Sci. 2008; 9: 1527-47.
2. Tamayo B, Alleyne BD, Djurovich PI, Lamansky S, Tsyba I, Ho NN, Bau R, T hompson ME. Synthesis and characterization of facial and meridional tris-cyclometalated iridium (III) complexes. J Am Chem Soc. 2003; 125(24): 7377-87.
3. McDonald AR, Lutz M, von Chrzanowski LS, van Klink GPM, Spek AL, van Koten G. Probing the mer-to fac-isomerization of triscyclometallated homo- and heteroleptic (C,N)3 iridium(III) complexes.Inorg Chem. 2008; 47: 6681-91.
4. Coughlin FJ, Westrol MS, Oyler KD, Byrne N, Kraml C, Zysman-Colman E, Lowry MS, Bernhard S. Synthesis, separation, and circularly polarized luminescence studies of enantiomers of iridium (III) luminop. Inorg Chem. 2008 47: 2039-48.
5. Baranoff E, Saurez S, Bugnon P, Barola C, Buscaino R, Scopeletti R, Zuperoll L, Graetzel M, Nazeeruddin MK. Sublimation not an innocent technique: A case of bis-cyclometalated iridium emitter for OLED. Inorg Chem. 2008 47: 6575-77.
6. Baranoff E, Bolink HJ, De Angelis F, Fantacci S, Di Censo D, Djellab K, Gratzel M, Nazeeruddin MK. An inconvenient influence of iridium (III) isomer on OLED efficiency. Dalton Trans. 2010; 39: 8914– 18.
7. Sivasubramaniam V, Brodkord F, Haning S, Loebl HP, van ElsbergenV, Boerner H, Scherf U, Kreyenschmidt M. Investigation of FIrpic in PhOLEDs via LC/MS technique. Cent Eur J Chem. 2009; 7(4): 836 –845.
Isomerization separation of cyclic metal ruthenium(III) complexes using ultra-high performance convergent chromatography system
Rui Chen and John P. McCauley
Figure 2 Ir(Fppy) 3 UPC 2 /UV chromatogram obtained using an ACQUIT Y UPC 2 2-EP3x100mm, 1.7 μm column. (A) Samples treated at 280 ° C for 24 hours; (B) Samples untreated at 25 ° C. The flow rate was 1.5 mL / min; the back pressure was 2175 psi; the 30% isopropanol auxiliary solution was isocratically eluted; the temperature was 40 °C. The data following the peak mark represents the relative percentage of each peak in terms of peak area.
Figure 3 UPC 2 /UV chromatogram of standard grade Flrpic. (A) An ACQUITY UPC 2 BEH 3x100mm, 1.7 μm column was used; the flow rate was 1.5 mL/min, the back pressure was 1740 psi, and 35% isopropanol was isocratically eluted at a temperature of 40 °C. (B) Two CHIRALPAKAS-H 4.6x150mm columns (5 μm each) were used. The flow rate was 3 mL/min, the back pressure was 2175 psi, and the 23% isopropanol co-solution was isocratically eluted; the temperature was 50 °C.
Figure 4: Acquired on an ACQUITY UPC 2 BEH3x100mm, 1.7μm column with isocratic elution (35% auxiliary solvent): (A) untreated Flrpic and (B) heat-stressed Flrpic UPC 2 /UV chromatogram. The flow rate was 1.5 mL/min; the back pressure was 2175 psi; the 35% isopropanol auxiliary solution was isocratically eluted; the temperature was 40 °C.
Figure 5 UV spectrum of a pair of Flrpic isomers.
Figure 6 was obtained using two CHIRALPAK AS-H 4.6 x 150 mm columns (each 5 μm): (A) untreated Flrpic and (B) UPC 2 /UV chromatogram of heat-stressed Flrpic. The flow rate was 3 mL/min, the back pressure was 2175 psi, and the 23% isopropanol co-solution was isocratically eluted; the temperature was 50 °C.