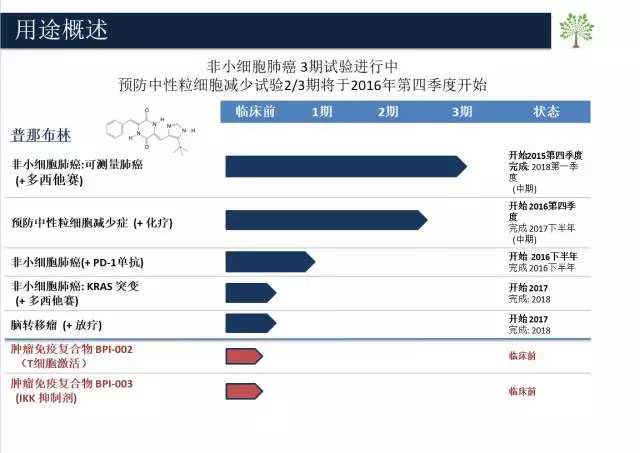
On March 10, Beyond Spring announced that it will be listed on NASDAQ in the United States under the ticker symbol “BYSI†for an issue price of US$20. It will initially publicize 174,286 common shares for public offering and private placement. Total revenue is expected to be $54.31 million. Since then, Wanchun Pharmaceutical has become the first biotech company in the US IPO this year. According to public information, Wanchun Pharmaceutical is a new drug research and development company founded by Dr. Huang Wei, a former Chinese “Thousand Talents Program†winner in Berkeley, Calif., with 1.1 innovative anti-tumor drugs (Punabrin). Plinabulin's Chinese and global patents. Together with Punebulin, Wanchun Pharmaceutical has a line of anti-tumor new drug development products consisting of multiple high-efficiency small molecules and peptide molecules. Wanchun Pharmaceutical's R&D pipeline (Source: Wanchun Pharmaceutical's official website) In 2007, Huang Wei began to intervene in the study of Pune Brin. In 2013, she formed Wanchun Pharmaceutical Co., Ltd. based in New York, San Diego and Dalian. With the company as the carrier, she mainly carried out research and development of Punebulin. Punebulin is an innovative drug with global independent intellectual property rights. It is a multi-targeted drug that can amplify immune cells by promoting dendritic cell maturation and activation of T cells related to tumor antigens. In addition, the tumor cells can be directly induced by activating the JNK channel; the tumor can be starved directly by inhibiting the blood flow of the tumor. Punebulin is the leading drug candidate for the treatment of Wanchun Pharmaceutical. Currently, a Phase III study for the treatment of non-small cell lung cancer (NSCLC) has begun, and plans to recruit 550 clinical trial patients from the United States, China, Australia and New Zealand. . Evaluation and enrollment of US patients have begun and additional centers have been added. Not long ago, CFDA has officially issued CTA (Clinical Trial Approval), allowing some research experiments in China. It is understood that the project will use punabulin combined with docetaxel to treat patients with advanced non-small cell lung cancer, in addition to the monotherapy effect of docetaxel. From the phase II clinical trial data, the median overall survival of docetaxel monotherapy was 6.7 months, and that of punebulin and docetaxel was 11.3 months. The OS was prolonged by 4.6 months, and its tumor efficiency was nearly doubled. The duration of remission (DOR) was 12.7 months. The DOR of docetaxel was significantly prolonged, which was statistically significant. The effect is very significant. In addition, the international multi-center phase II and III clinical trials of Punabrin injection for the prevention of chemotherapy-induced neutropenia have been initiated in September 2016 with the FDA. The FDA has agreed to start the trial, China International The multi-center, phase III clinical application data has been reported to the CDE and is expected to be approved and clinical trials will begin next year. We are a specialized manufacturers of Animal Extract from China; We support Animal Organ Extract, Animal Protein Powder, Velvet Antler Extract, Deer Whip Extract, Marine Fish Collagen and so on. With advanced R & D and manufacturing in Animal Extract. With high-quality products of Animal Extract Raw Material, we can be a trust suppliers / factory. As a wholesale of Animal Extract, we have the perfect after-sales service and technical support. Look forward to your cooperation! Velvet Antler Extract,Animal Organ Extract,Deer Whip Extract,Marine Fish Collagen,Animal Protein Powder Xi'an Quanao Biotech Co., Ltd. , https://www.quanaobio.com