2. CloneSelect Imager (Molecular Devices, LLC) 3. CloneMedia Semi-Solid Media for hybridomas/myelomas (Molecular Devices, LLC Cat #K8600/K8610) 4. CloneDetect Reagent, Mouse IgG (H+L) Specific, Fluorescein (Molecular Devices, LLC Cat #K8220) Non-Woven Protective Products,Non Woven Coverall,Disposable Non Woven Isolation,Disposable Waterproof Isolation Gown Ningbo Carest Medical Instrument Co.,ltd , https://www.carestmed.com


ClonePix combines CloneSelect Imager technology to enhance the development of virus-specific hybridoma cells
Disease drug specific hybridoma
The ClonePix system was used to screen hundreds of subclones from high-throughput maternal hybridoma cells (historical data suggest that maternal cell lines yield less than 1 mg/L of monoclonal antibody). Viable cells secreting high IgG were further evaluated using the CloneSelect Imager system which objectively evaluated cell growth. Efficient automated clonal selection techniques aim to recapture high titer hybridoma cell lines that produce antibodies with high specificity for antigens. The ClonePix and CloneSelect Imager technologies provide sufficient antibodies to viral antigens for the development of immunodiagnostic techniques.
material
1. ClonePix System (Molecular Devices, LLC)
Rescuing low-yield hybridoma cells
Traditional hybridoma development methods involve a chemically mediated fusion process of myeloma cells and antigen-immunized host spleen cells. The fused hybridoma cells undergo a selection process known as the long and inefficient limited dilution method, followed by screening by ELISA to isolate a limited number of antigen-specific monoclonal hybridoma cells. Too long a timeline (more than 30 weeks) and a large number of treatments allow the limiting dilution method to produce low titer cell lines that do not accurately quantify activity and productivity.
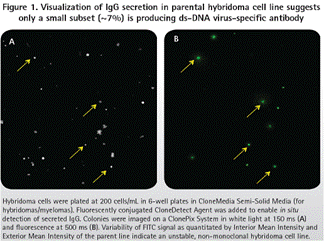
The earliest double-stranded DNA virus-specific hybridoma cell lines were developed by the limiting dilution method in 1990s, but the hybridoma cells screened were not very good in monoclonality and stability. Repeated cultures (static and rotating) were performed for many years with different serum and medium components, and these clones showed instability (Fig. 1). Moreover, the yield of antibodies never exceeds 3 mg/L. Due to the vastly different aggregates that can be observed in preps, the quality of purified IgG is difficult to maintain consistent.
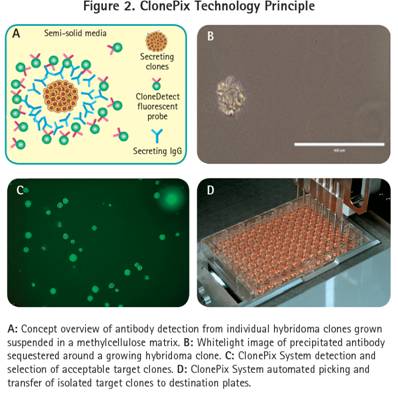
The specific hybridoma cell line is recovered and stabilized by subcloning the parental cell line of the high titer antibody. The ClonePix technology platform re-screens a large number of previously fused hybridoma cells using semi-solid media and CloneDetect label-free detection technology (Figure 2), showing significant technical advantages.
Rapid isolation of high-yielding clones secreting antibodies
The ClonePix system shows significant improvements in process efficiency and cost savings, increasing the probability of finding rare secretors. Therefore, the time of production of the ClonePix2 technology monoclonal antibody is reduced to half of the limiting dilution method.
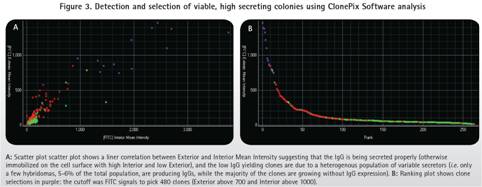
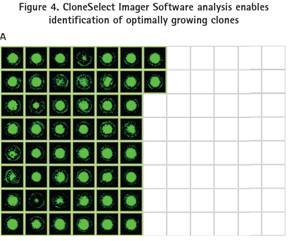
In this process, the number of parental hybridoma clones is first amplified and then ClonePix analyzes and selects the desired clone. The ClonePix system images and analyzes fluorescence intensity using the FITC-labeled CloneDetect reagent. FITC-positive clones were ranked according to the average fluorescence intensity of the clones from high to low. In summary, only 5-6% of positive clones of 480-FITC were picked. Most highly secreted clones are smaller (40-60 cells) than large, fast-growing negative clones (70-150 cells) (Figure 3).
480 high-yielding clones were picked from semi-solid medium into five 96-well microplates containing 200 μL of liquid medium and cultured for 3 days. For objective and quantitative assessment of cell numbers, clones in 96-well plates were imaged using the CloneSelect Imager system. Ultra-fast imaging speed (96-well plates less than 90 sec) enables rapid monitoring and evaluation of 5-day clone growth. The first 146 fast-growing clones were isolated by efficient imaging with CloneSelect Imager (Figure 4).
Screening and identification of subclones for pilot scale analysis
Clones of the selected pre-146 growth rate were subjected to antigen specificity analysis using a synthetic polypeptide having an antibody epitope. Polypeptides and antibodies show strong affinity and are fully consistent with the interpretation of previous studies. Therefore, synthetic peptides are used to screen for new subclones with high antibody affinity.
The biotin-labeled polypeptide was loaded on the surface of a streptomycin-containing microplate, and 146 undiluted hybridoma cell supernatants were added to determine the binding ability of IgG. The first seven special subclones showing the optimal binding force were selected.
Pilot scale production of high IgG secreted subclones
Seven high-value specific subclones were produced in large scale in liquid medium. These clones were amplified and once sufficient medium volume was obtained, 5 subclones were frozen for future research reference. The remaining 2 high-affinity/high-IgG secreted clones continued to expand for 2-3 weeks until the pilot production (1 liter) was completed.
Workflow to save low-yield hybridoma cells
To identify subclones of double-stranded DNA virus-specific antibodies used to develop advanced diagnostics, ClonePix technology screened 480 FITC-positive hybridoma clones based on semi-solid media technology and FITC-labeled CloneDetect reagent. CloneSelect Imager identified 146 of the rapidly produced clones, and 7 high-yield clones were confirmed to be highly specific to the target polypeptide. Two of the subclones were selected for pilot production and the remaining five were used as cell banks. This procedure reduced the screening time of hybridoma cells up to 50% of the screening time of conventional limiting dilution methods, and screened robust target cloned cells for additional research and production.
Monoclonal validation and homogenization of IgG secretors
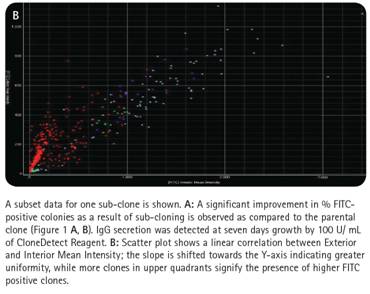
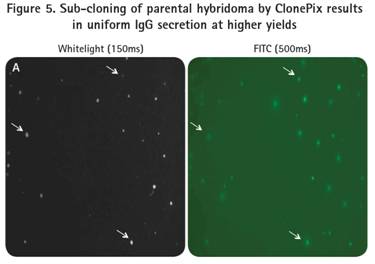
After cloning and specific confirmation of the ClonePix system, the two subclones were amplified and re-planted into 6-well plates of semi-solid medium with CloneDetect reagent. Imaging and analysis of the ClonePix system indicated that the clones normally secreted IgG, and that clones with high production of IgG were observed by the homogeneous homogenization process (Fig. 5).
Production capacity of subclones of novel double-stranded DNA virus hybridoma cells
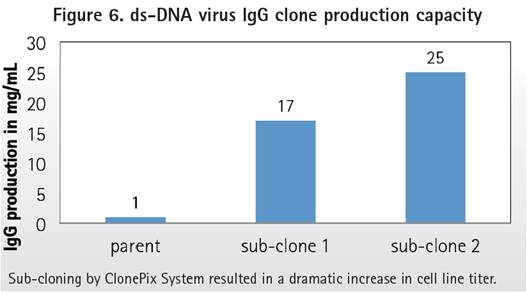
Re-screening low yield parental clones using ClonePix technology. High-yield, stable subclones were recovered. Protein G purified monoclonal antibody to quantify total yield. Compared to the historical yield of parental clones (~1 mg/L), the yield of new subcloned cell IgG was significantly increased (17-25 mg/L), as shown in Figure 6.
in conclusion
ClonePix technology improves screening time periods and quality of subcloned hybridoma cells, reducing manual handling. Moreover, CloneMatrix Media and CloneDetect reagents increase the growth and detection sensitivity of secreted clones. ClonePix data indicates that the low productivity of the parent is due to the small number of cells producing antibodies being pressed by cells that are fast, antibody-free.
Compared to the production of parental hybridoma cells (05-1 mg/L), combined with the use of CloneSelect Imager, the ClonePix system proved to be a powerful tool for identifying high-yield (17-25 mg/L), high-affinity hybridoma cell subclones. Therefore, this technology contributes to the advancement of new therapeutic antibodies specific for double-stranded DNA viruses.
For more information, please contact AbiPointe Biotechnology.