Cosmetics are a mixture of various raw materials processed by reasonable allocation. There are many kinds of cosmetics with different properties. According to the cosmetic raw material performance and use, can be divided into two categories of substrate raw materials and auxiliary raw materials. The former is a kind of main raw material of cosmetics, which occupies a large proportion in cosmetics formula and plays a major role in cosmetics. The latter play a role in shaping, stabilizing, or imbuing cosmetics with color, fragrance and other properties that are extremely important in small amounts in cosmetic formulations. Cosmetics is with natural, synthetic or extract all sorts of action different material as raw material, via heating, agitation and emulsification wait for production program processing and become chemical mixture material.
tryptamine labs review,tryptamine supplement,tryptamine high Shaanxi YXchuang Biotechnology Co., Ltd , https://www.peptidenootropic.com
ACQUITY UPLC ® HSS Columns
The simplest type of polymer is an addition polymer. The addition polymer is formed by sequential addition of monomer units without losing any molecules. The polycondensation polymer is formed by a polycondensation reaction of two or more different monomers in which individual molecules are bonded together and produce by-products such as water. In the polymerization reaction, individual molecules can not only bind linearly to each other, but also form branched isomers. Since polymers can form a variety of isomers, their isolation and identification can be particularly difficult. In addition, degradation products and by-products are formed under polymerization conditions, which are also required to be identified. The properties of polymeric materials can be affected by the distribution of isomers and oligomers.
In this application note, we investigated various addition polymers such as polystyrene (PS) and polymethyl methacrylate (PMMA) to evaluate the separation range of UPC 2 . The information obtained is then used for polycondensation copolymers analyzed by MS and UV detection, such as bisphenol A formal polycondensation polymer (PBAA) and poly[(phenyl glycidyl ether)-co-formaldehyde (PGEF). 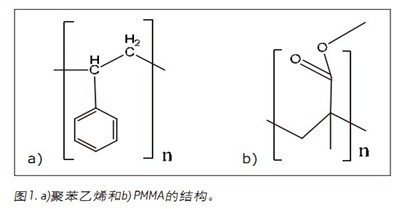
Case study 1 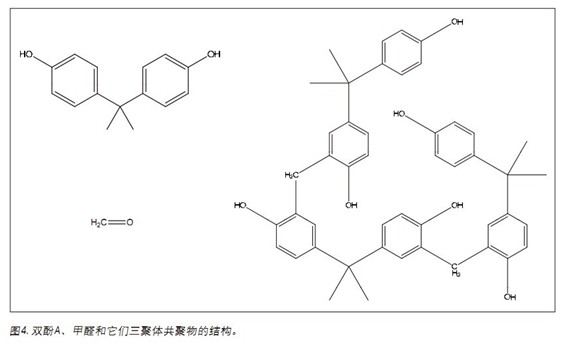
In this case, MS provides valuable information for the initial material in the polymerization that is not involved in the reaction. This analytical method can be used for reaction monitoring to ensure complete utilization of the initial material.
Case study 2 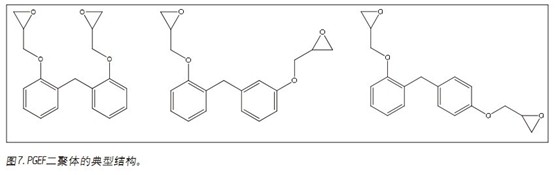

Since these isomers are much lower in the sample than the dimers and trimers, they are ignored by the UV detector.
In this case, UPC 2 equipped with an MS detector provides detailed information about the isomers present in the polymer sample. Data on degradation products in the sample helps to adjust the polymerization conditions to prevent loss or alteration of the glycidyl ether chain. If required, the analyst can separate the individual isomers and analyze them by structural identification methods such as NMR to determine the exact location of the bonds.
in conclusion
references
UPC
Baiba Cabovska and Michael O'Leary
Waters Corporation (Milford, MA, USA)
Application advantage
â– compared to GC, ACQUITY UPC 2 â„¢ analysis may be thermally labile polymer having a higher molecular weight.
â– ACQUITY UPC 2 is capable of analyzing polar and non-polar polymers.
■Supercritical fluid mobile phase and sub-2 μm particle phase stationary phase can shorten the retention time of large molecular weight compounds.
â– ACQUITY UPC 2 uses less toxic solvents than normal phase LC.
â– MS can provide supplemental information for UV data for individual oligomer identification, impurity determination and formulation analysis.
Waters Solutions
ACQUITY UPC 2 with PDA and ACQUITY ® SQD
ACQUITY UPC 2 BEH Column
Empower ® 3 CDS
Key words
Polymer, UPC 2 , supercritical fluid, SFC, polystyrene, PMMA, convergent chromatography, oligomer
Introduction
The most common polymer analysis is the use of gel permeation chromatography (GPC) to determine the average molecular weight and polydispersity. However, other analytical techniques 1-4 will be used when high resolution separation of various oligomers is required to assess material properties or to understand polymer structures. Low molecular weight polymers can be analyzed by liquid chromatography (LC), gas chromatography (GC), and supercritical fluid chromatography (SFC). The choice of separation technique will generally depend on solubility, average molecular weight, and polymer thermal stability. Waters ® UltraPerformance Convergence ChromatographyTM (UPC2 ® ) is an innovation in SFC technology that offers several advantages in the separation of complex oligomer materials. Since supercritical carbon dioxide has a lower viscosity than liquid, higher flow rates can be achieved, resulting in shorter analysis times than LC. Convergence chromatography, which operates at lower temperatures than GC, can be used to analyze thermally unstable materials. In addition, UPC 2 is able to separate higher mass non-volatile oligomers compared to GC. Another advantage is that a sub-2 μm particle column can be used to obtain a higher theoretical plate number and better resolution than conventional SFC. When the polymer has a chromophoric group, UV detection can be used. If information about the molecular weight of the isomer is required, it can be detected using a mass spectrometer (MS). UPC 2 can be used in series with UV and MS detectors.
experiment
Sample Preparation
All polymer samples were dissolved in tetrahydrofuran (THF) at a concentration of 10 mg/mL.
UPC 2 conditions
System: ACQUITY UPC 2 with PDA and ACQUITY SQD
Mobile phase A: CO 2 (food grade)
Mobile phase B: 0.3% ammonium hydroxide in methanol solution
Column temperature: 60 °C
Injection volume: 1.0 μL
MS ionization mode: ESI (+ or -, depending on the sample)
MS scan range: 150 to 2000 m/z
Capillary voltage: 1 kV
Taper voltage: 25 V
Compensation solvent: 0.3% ammonium hydroxide methanol solution
ABPR: See specific sample
Flow rate: See specific sample
Vial: 12 x 32 mm clear glass threaded neck vial with a volume of 2 mL
Data Management: Empower 3 CDS
Results and discussion
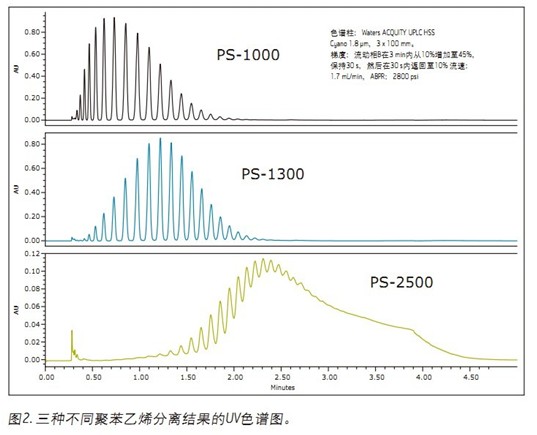
Various molecular weight polystyrene and PMMA (Figure 1) were evaluated using UPC 2 with a sub-2 μm particle size column. Figure 2 shows the separation of three different polystyrenes. Separation of all PS-1000 and PS-1300 oligomers was completed in 2.5 minutes. However, the PS-2500 only achieved partial separation. As the molecular weight increases, the complexity of the polymer increases accordingly, so that baseline separation cannot be achieved.
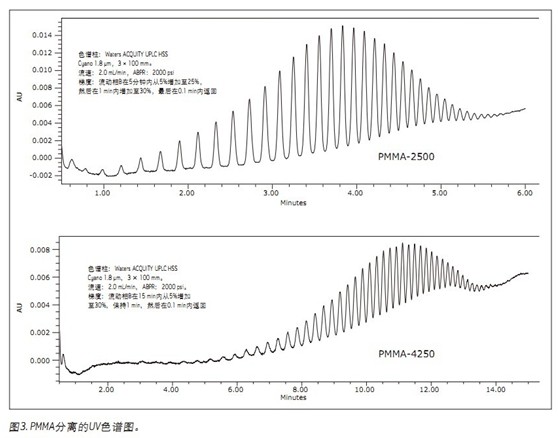
As shown in Figure 3, PMMA oligomers can be separated at higher masses than polystyrene. As the average molecular weight of the polymer increases, the retention time required for complete elution also increases accordingly. The molecular weight range of the polymer that UPC 2 can analyze depends on the solubility of the sample in CO 2 , the type of polymer, and the length of time that the analyst can accept to achieve the separation. High molecular weight polymers typically require a higher concentration of organic cosolvent to elute the column. However, increasing the concentration of the organic co-solvent also leads to an increase in back pressure. To keep the back pressure within an acceptable range requires a reduction in flow rate, which ultimately results in an increase in run time.
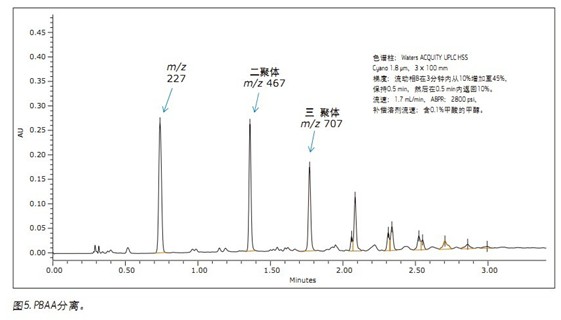
The role of UPC 2 in polymer analysis will be reflected in the following two case studies. The first case involved the analysis of a bisphenol A formaldehyde polycondensation copolymer (PBAA), as shown in Figure 4. The copolymer is formed by addition of polybisphenol A to formaldehyde, during which water is formed. The PBAA was analyzed and the expected dimers, trimers, and subsequent oligomer peaks were observed. However, a distinct peak shape of m/z 227 was observed at a retention time of 0.7 min (Fig. 5). The initial compounds of the polymer are bisphenol A and formaldehyde. m/z 227 (ESI-) corresponds to the bisphenol A molecular ion [MH]-.
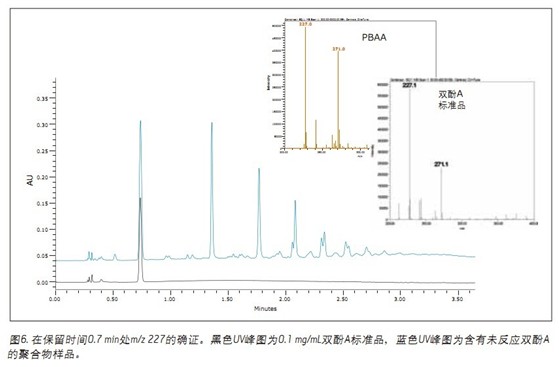
Unreacted bisphenol A was confirmed by UV and MS detection using a corroborating standard (Figure 6). The retention time of the bisphenol A standard is consistent with the unknown peak in the polymer sample. Moreover, the MS spectrum of bisphenol A also matches the spectrum of the target peak. The formic acid adducts also appear in the mass spectrum and provide additional evidence for this.
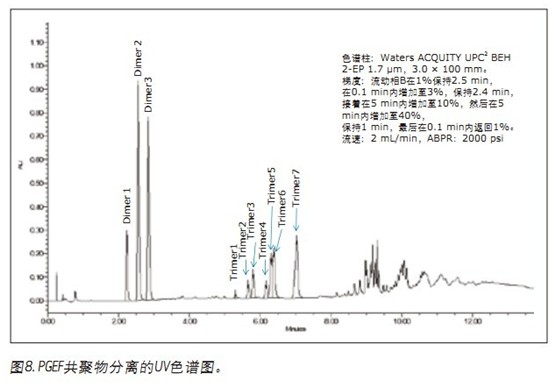
The second case involved the analysis of poly[(phenylglycidyl ether)-co-formaldehyde] (Figure 7). As shown in Figure 8, the individual isomers of the dimer are easily separated. Depending on the structure of the starting molecule, there are three possible positions with which the next unit can be combined. For dimers, this means that there may be six different isomers in the sample, but only three are observed. For trimers and subsequent oligomers, the possible structure increases exponentially. In this separation, a total of seven trimers were isolated.
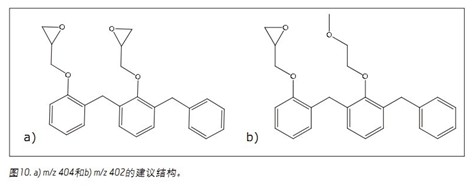
When viewing the same separated total ion chromatogram (TIC) (Figure 9), additional peaks were observed between the dimer and the trimer. The observed ion clusters have m/z ratios of 404 and 402. These masses may be due to changes in the structure of the phenyl glycidyl ether, either by the loss of a glycidyl ether chain or by the ring opening of the ether ring (Figure 10). Oligomers comprising the degradation unit are then also present between the trimer and the tetramer.
UPC 2 /MS is a powerful tool for identifying complex oligomer materials. A wide range of selectivities facilitates the separation of similar compounds, such as isomers of oligomers. Other advantages include suitable for polar and non-polar polymers, lower analytical temperatures, and a greater mass range than GC. The use of a supercritical fluid mobile phase can shorten the retention time of large molecular weight compounds compared to LC. Additional MS detection provides additional information for UV data and can be used for reaction monitoring, identification of individual oligomers, impurity determination, and formulation analysis.
1. Ibanez E, Senorans FJ. Tuning of mobile and stationary phase polarity for the
Separation of polar compounds by SFC. J Biochem Biophys Methods. 2000; 43: 25-43.
2. Hoffman BJ, Taylor LT, Rumbelow S, Goff L, Pinkston JD. Separation of
Derivatized alcohol ethoxylates and propoxylates by low temperature
Column supercritical fluid chromatography using ultraviolet absorbance
Detection. J Chromatogr A. 2004; 1034: 2007-212.
3. Hanton SD. Mass Spectrometry of Polymers and Polymer Surfaces. Chem Rev.
2001; 101: 527-569.
4. Takahashi K. Polymer analysis by supercritical fluid chromatography.
J Bioscience Bioeng. In press, available online 5 March 2013.