The topic of this issue is to discuss the extraction of ribonucleic acid (RNA). It may be questioned in your mind: RNA extraction is not very simple? Just follow the standard steps, add one plus reagent to complete Is it necessary to pay more attention to the opening of the article to talk about this matter? In fact, the simpler things are more difficult to solve when encountering difficulties, please look at the temperament, listen to us. Lab's experience and customer's problems <br>With the long-standing experience of Hualian Genomics Laboratory in undertaking client RNA samples, we often encounter problems such as (1) insufficient total RNA, (2) RNA There are serious salt pollution, (3) RNA has serious organic solvent pollution, (4) DNA contamination and (5) severe degradation of the sample (Figure 1 Figure 2). The first four problems are solved, and only need to be extracted again or purified, but the sample degradation is more difficult. It is often because of re-preparation of the sample, which consumes a lot of effort and time, and finally does not take it. Good results. What are the methods of RNA extraction? How is the appliance cleaned? How is the sample collected/saved? How to break and homogenize the sample? Centrifugal stratification of samples Precipitation of RNA samples Cleaning of RNA samples to remove salts <br>In order to remove salts from RNA precipitates, we can wash them with 70~75% ethanol. If possible, try to suspend the nucleic acid precipitate. It is best to use two washes. The centrifuge tube was centrifuged in a centrifuge, and the residual ethanol was carefully sucked off with a pipette, and the residual nucleic acid was slightly drained or dried to volatilize the remaining ethanol, and then reconstituted with DEPC water. For more information about Hualian products, please visit  Turn Style Gate,Full Height Turnstile Gate,Pedestrian Turnstiles,Sliding Turnstile Shenzhen Unisec Technology Co.,ltd , https://www.uniqscansecurity.com
Customers often ask why performing RNA experiments require RNA quality so that other methods such as Real-time Quantitative Polymerase Chain Reaction (Q-PCR) do not.
Before answering this question, we must start from the principle of experiment. Simply speaking, the principle of Q-PCR detection is to design a pair of gene-specific primers for the gene to be detected, and to carry out reverse transcription reaction (RT) with PCR. The method of amplifying and detecting the amplified product in time is used to push back the original RNA content; while the RT method mainly uses a random primer (Random_primer) to perform the recombination of complementary DNA (cDNA), so even if the RNA is slightly cleaved, The template RNA can also be faithfully turned into cDNA. However, the chip experiment is carried out mainly by amplifying the complementary RNA (cRNA) which is completely paired with the message RNA (mRNA), and then performing the hybrid reaction. This step must utilize the poly(dT)-T7 promoter sequence and the poly(A) of the message RNA. After binding, the T7 promoter sequence is ligated into the 5th end of the reverse transcribed cDNA, and then the T7 promoter is used to drive the in-tube reverse transcription reaction to synthesize the anti-strand cRNA, and then the subsequent chip hybridization experiment is carried out. . It is conceivable that if RNA is degraded severely, it means that many mRNAs are not faithfully amplified and thus produce many false negative results. At this point, readers may have a concept in mind. It is assumed that Q-PCR and chips are used in the storyline of the scorpion. If people with Q-PCR can touch the elephant's ear, they can say that there is an elephant. By counting the number of ears, you can count the number of elephants; but those who use the chip test method must first confirm that the individual they touch has the complete characteristics of the elephant, in order to be sure that there is an elephant here. This also explains why the chip experiment requires the quality of RNA. 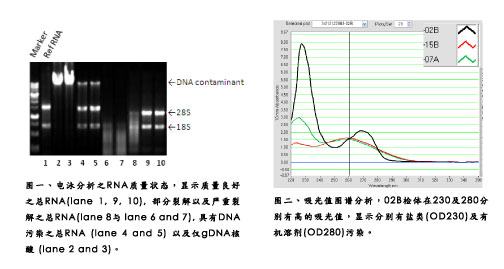
In addition to traditional purification methods, such as LiCl precipitation, in response to the huge demand for RNA research, there are many well-organized RNA extraction kits available on the market to provide research for different extraction needs. The design principle of these products is nothing more than The organic solvent is used for layered extraction, such as: (1) phenol-chloroform; or the use of RNA in the presence of hydrophobic properties in alcohol, adsorption separation such as: (2) adsorption column (Silica column). In general, as long as you have the correct RNA manipulation concept and the correct steps, you can extract good quality RNA in general, but the distribution of RNA composition obtained by different extraction methods will be different, so it is recommended to be based on the analysis. For RNA, select the correct extraction method or kit. For example, if you want to study the performance of small RNA, you can't choose the method of extracting mRNA. In addition, samples containing high levels of endogenous ribozyme degrading enzyme (RNase) are recommended to use phenol-chloroform-containing lysates to increase the ability to remove enzyme activity. Below I take TRIzol's operation steps as an example to share with readers the precautions in the RNA extraction step and the methods to improve the extraction of RNA values ​​and quantities.
The biggest enemy in RNA extraction is RNase, a very tenacious RNA-degrading enzyme that is almost ubiquitous, with endogenous RNase in our environment, on the body surface and even in cells. RNase does not require coenzymes or cofactors when exhibiting activity. Although it loses its activity in the environment of denaturing solutions, it still shows some activity when denaturing substances are removed, even in low concentrations of SDS solution. It is active, so it can effectively eliminate the pollution of RNase, and the extraction of RNA is half successful. Therefore, the first task before the operation is to dry all the instruments at 180 ° C for 6 hours or more; or immerse them in 3% H 2 O 2 for more than 30 minutes, then rinse them with decontaminated DEPC water. It is used after being sterilized by aluminum foil. It is necessary to operate in a clean, dust-free environment when operating the extraction. If the environment permits, it is best to operate in a biological cabinet or a chemical cabinet.
Earlier we mentioned that there are endogenous RNases in cells, and their expression varies greatly among different types of tissues. For example, brain tissue and heart tissue have relatively low endogenous RNase, but thymus tissue and pancreatic tissue retain about 100,000 times the endogenous RNase of brain tissue. Therefore, animal tissues and the like are preferably first frozen with liquid nitrogen, and then subjected to subsequent treatment. It is not recommended to directly throw them into the -80 °C refrigerator. Because rapid freezing can freeze the activity of RNase, but -80 °C freezing is a slow cooling, it will still cause partial RNA degradation; if you can not quickly freeze the sample, you can also use the commercially available sample preservation solution, can also achieve good results .
Regarding the fragmentation and homogenization of the sample, the focus of this step should be on how to allow the organic lysate to quickly infiltrate the sample. The phenol-chloroform can dissolve the cells, and the associated protein will cause protein denaturation, so the activity of the endogenous RNase will go. Activation; on the contrary, if the cells cannot be lysed rapidly and the protein is denatured and denatured, RNase will degrade RNA. Therefore, how to rapidly lyse different tissue cells is a subject worthy of consideration and adjustment, and it is also the key to success. As far as the type of the sample is concerned, in general, the cultured cells are mostly monolayer cells, and the extraction is relatively easy, but the extraction of the three-dimensional tissue encounters the problem of uneven homogenization and causes RNA degradation. Therefore, low-temperature liquid nitrogen milling is the most effective way to break the tissue, but liquid nitrogen milling is more troublesome. If you encounter a large number of samples, it will take time and effort, but if conditions permit, this is still a good idea. But considering efficiency and speed, homogenizer is a better choice, but the process of using homogenizer has several important points to note: (1) speed is fast, complete cell homogenization before RNase exhibits activity, (2 Avoid heat production to avoid degradation of RNA, (3) Do not over-grind, excessive grinding will break the DNA, causing DNA contamination of small fragments. In addition, there is a neglected focus in the process of homogenizing lysed cells. The ratio of general cells to organic lysates is often neglected. Some operators will think that a large amount of RNA can be obtained by putting more tissue, but no Realizing that too much tissue and too little lysate caused the cells to be completely lysed, the RNase could not be completely denatured, but the effect was counterproductive, and finally the RNA was severely degraded. Therefore, if the homogenate is too viscous and cannot be stratified after the reaction, the lysate must be added.
In the extraction method of phenol-chloroform, the centrifugal rotation number of the mixed liquid cannot be arbitrarily adjusted, and should be fixed at the number of revolutions of 12000 g to avoid the inability to effectively stratify due to the change of sedimentation coefficient. When the centrifugation is completed, it can be clearly observed that the sample is divided into the upper water layer and the lower organic layer, and the RNA we want is dissolved in the upper water layer, and the volume is about 500-600 μl. At this point it is recommended to use a small-diameter 100 or 200μl tip to pipette the aqueous solution into a clean 1.5ml centrifuge tube. The common mistake in this step is that the operator is eager to remove the water layer and agitate the original layer; Or the sensory extraction is not easy to recover more RNA, so the water layer is taken out as much as possible, thus causing the absorption of organic solution and a large amount of DNA contamination (Fig. 3), causing troubles in subsequent experimental treatments and even experimental failures. It is recommended to extract only 7~8% of the RNA aqueous solution for the next step. As the saying goes, there is a need for it. You don't have to give up the whole forest for a tree. The timely selection can ensure the follow-up experiment goes smoothly. In addition, in general, phenol and water have a certain proportion of mutual solubility, so there is still a certain phenol residue in the aqueous solution of RNA at this time, it is recommended to use chloroform to carry out a centrifugal stratification to remove residual phenol. 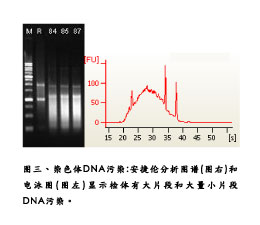
Generally, we use ethanol (aqueous solution: ethanol = 1:2.5) or isopropanol (aqueous solution: isopropanol = 1:1) for RNA precipitation. There is no significant difference in effect, but if the RNA content is known to be less The ethanol precipitation method can be used, and the sample is placed in a refrigerator at -80 ° C for one night and then centrifuged, and with the increase of the centrifugation time, thus increasing the recovery rate of the RNA precipitate.
Conclusion <br>If you noticed improvements in the above sections, but still can't extract good quality RNA, then we may have to check whether our experimental design will lead to the activation of RNA degradation mechanisms, such as: The cell is undergoing a physiological reaction of apoptosis or tissue necrosis; or the treated drug directly causes RNA degradation, etc., if it is likely to be experimental design correction or down-regulation of RNA quality requirements.
The above techniques for RNA extraction are the practical experience of Hualian Genome Laboratories operating tens of thousands of various RNA samples over the years. The accumulated experience is for some experienced readers. It may be that the wild man offered it. However, I hope that such experience sharing can help those customers who have encountered difficulties in preparing for RNA, and can successfully complete the preparation of RNA samples. The laboratory is committed to the spirit of mission and takes every delivery to us seriously. The sample on hand will be sent to the client in the hands of the client with the fastest results. If there is anything incomplete or unclear about the above content, please feel free to contact us or discuss with Hualian Lab. We will do our best.