FD Chicken Vegetable Fruit Mixed Cube,FD Apple,FD Kiwifruit,FD Chicken Carrot Cube,FD Salmon with Carrot Cube FD Chicken Vegetable Fruit Mixed Cube,FD Apple,FD Kiwifruit Eastan Pet , http://www.eastanpet.com
Structure:Disposable infusion pump is consisted of silicone reservoir, filter, connector, tube, PCA, etc.
 Number of Registered Certificate: 15041892 001
Operative Standard:Â EN ISO 13485:2012+AC:2012, MDD 93/42/EEC Annex II Article 3
Expected Usage:Â The product works as the persistent and default speed of charge. It's driven by silicone reservoir, it realizes minim and persistent charge by controlling the flow rate using current limiting device. Patient can append dose by medical order with the help of PCA, which realize the goal of clinical minim charge.
CharacteristicsThe raw material of disposable infusion pump has no side effects to human body.
Using Instructions
1) Open the package after confirming that the package is in good condition and the type as well as specification is correct.
2) Close the clip and then open the shell of the charge orifice, absorb the deployed liquid medicine into the asepsis injector.
3) Move the card in the automatic controller when you use the type of CBI+PCA-M200.
4) Open the clip to drain the air away, connect the pipe joint after confirming that some liquid outflows.
Points of Attention
1) Read the instruction carefully before use.
2) Check whether the package is in good condition, or else it is strictly prohibited to use.
3) It is only for single use, discard after use.
4) The product was sterilized by epoxy ethane and assures asepsis for two years.
5) The operator must obey the sterilized operating condition.
6) Volume can't exceed 10% of the max volume marked on reservoir, or it may blow out.
7) Its nominal flow rate was evaluated by using water for injection .
8) The product is deposited under door temperature in dry condition.
9) Adrenalin and CAT (calcium antagonists) are strictly prohibited for use.
10) The product contains DEHP.
11) Clinical medical staff should take substitute Product to avoid possibility toxicity may arise on those high-risk crowd (new arrivals, adolescent males, pregnant/nursing women).
12) The product is not suitable for infusion by using fat-soluble drugs (e.g. Fat Emulsion).
13) Based on both domestic and overseas research materials, clinical medical staff should avoid PVC tube and drugs to be militated, the product is not available for drugs which are incompatible with PVC tube.
Package
Individual disposable infusion pump is packed by blister packing, the middle and outer package is corrugated box.
Storage
The products should be stored in less than 80% relative humidity, non-corrosive gases and well-ventilated room.
Description of Label
1. for single use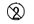
2.The product is EO sterilized
Â
Sterile Model:EO sterilization   Â
Validated period of Sterilization:Two years
Manufacturing Company:Changzhou Medical Bioengineering Co., Ltd
Address:Tianzhutang, Dongqing, Zhenglu Town, Wujin District, Changzhou City, 213114, China
Post Code:213114
Tel:86-519-88966750
Fax:86-519-88964890
Website:www.china-changsheng.com
EU Representative:Shanghai International Holding Corp. GmbH (Europe)Â JIN LIANG
Address:Eiffestrasse 80, 20537 Hamburg, Germany
Type
 Specification
 Max Capacity
(ml)
 Flow Rate
(ml/h)
 Bolus
(ml/time)
 Time Lock-out
(min)
Â
With bolus
 CBI+PCA-M100
 100
 2.0\3.0\4.0
 0.5
 15
Â
CBI+PCA-M200
 200
 2.0\3.0\4.0
 0.5
 15
Â
CBI+PCA-M275
 275
 2.0\3.0\4.0\5.0
 0.5
 15
Â
Without bolus
 CBI-M100
 100
 2.0\3.0\4.0
 Â
 Â
Â
CBI-M200
 200
 2.0\3.0\4.0
 Â
 Â
Â
CBI-M275
 275
 2.0\3.0\4.0\5.0
 Â
 Â
Â
Multirate
 CBI+PCA-MR100
 100
 2,4,6, 8
 0.5
 15
Â
CBI+PCA-MR200
 200
 2,4,6, 8
 0.5
 15
Â
CBI+PCA-MR275
 275
 2,4,6, 8
 0.5
 15
Â
Structure:Disposable infusion pump is consisted of silicone reservoir, filter, connector, tube, PCA, etc.
 Number of Registered Certificate: 15041892 001
Operative Standard:Â EN ISO 13485:2012+AC:2012, MDD 93/42/EEC Annex II Article 3
Expected Usage:Â The product works as the persistent and default speed of charge. It's driven by silicone reservoir, it realizes minim and persistent charge by controlling the flow rate using current limiting device. Patient can append dose by medical order with the help of PCA, which realize the goal of clinical minim charge.
CharacteristicsThe raw material of disposable infusion pump has no side effects to human body.
Using Instructions
1) Open the package after confirming that the package is in good condition and the type as well as specification is correct.
2) Close the clip and then open the shell of the charge orifice, absorb the deployed liquid medicine into the asepsis injector.
3) Move the card in the automatic controller when you use the type of CBI+PCA-M200.
4) Open the clip to drain the air away, connect the pipe joint after confirming that some liquid outflows.
Points of Attention
1) Read the instruction carefully before use.
2) Check whether the package is in good condition, or else it is strictly prohibited to use.
3) It is only for single use, discard after use.
4) The product was sterilized by epoxy ethane and assures asepsis for two years.
5) The operator must obey the sterilized operating condition.
6) Volume can't exceed 10% of the max volume marked on reservoir, or it may blow out.
7) Its nominal flow rate was evaluated by using water for injection .
8) The product is deposited under door temperature in dry condition.
9) Adrenalin and CAT (calcium antagonists) are strictly prohibited for use.
10) The product contains DEHP.
11) Clinical medical staff should take substitute Product to avoid possibility toxicity may arise on those high-risk crowd (new arrivals, adolescent males, pregnant/nursing women).
12) The product is not suitable for infusion by using fat-soluble drugs (e.g. Fat Emulsion).
13) Based on both domestic and overseas research materials, clinical medical staff should avoid PVC tube and drugs to be militated, the product is not available for drugs which are incompatible with PVC tube.
Package
Individual disposable infusion pump is packed by blister packing, the middle and outer package is corrugated box.
Storage
The products should be stored in less than 80% relative humidity, non-corrosive gases and well-ventilated room.
Description of Label
1. for single use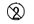
2.The product is EO sterilized
Â
Sterile Model:EO sterilization   Â
Validated period of Sterilization:Two years
Manufacturing Company:Changzhou Medical Bioengineering Co., Ltd
Address:Tianzhutang, Dongqing, Zhenglu Town, Wujin District, Changzhou City, 213114, China
Post Code:213114
Tel:86-519-88966750
Fax:86-519-88964890
Website:www.china-changsheng.com
EU Representative:Shanghai International Holding Corp. GmbH (Europe)Â JIN LIANG
Address:Eiffestrasse 80, 20537 Hamburg, Germany
Type
 Specification
 Max Capacity
(ml)
 Flow Rate
(ml/h)
 Bolus
(ml/time)
 Time Lock-out
(min)
Â
With bolus
 CBI+PCA-M100
 100
 2.0\3.0\4.0
 0.5
 15
Â
CBI+PCA-M200
 200
 2.0\3.0\4.0
 0.5
 15
Â
CBI+PCA-M275
 275
 2.0\3.0\4.0\5.0
 0.5
 15
Â
Without bolus
 CBI-M100
 100
 2.0\3.0\4.0
 Â
 Â
Â
CBI-M200
 200
 2.0\3.0\4.0
 Â
 Â
Â
CBI-M275
 275
 2.0\3.0\4.0\5.0
 Â
 Â
Â
Multirate
 CBI+PCA-MR100
 100
 2,4,6, 8
 0.5
 15
Â
CBI+PCA-MR200
 200
 2,4,6, 8
 0.5
 15
Â
CBI+PCA-MR275
 275
 2,4,6, 8
 0.5
 15
Â
Disposable Infusion Pump (CBI+PCA-M100)
Model NO.: CBI+PCA-M100
Nominal Volume: 100
Mean Flow Rate(Ml/H): 2.0/3.0/4.0
Self-Control Dosage(Ml/Time): 0.5
Bolus Refill Time: 15
Trademark: CHANGSHENG
Specification: ISO/CE
Origin: China
HS Code: 9018907000
Model NO.: CBI+PCA-M100
Nominal Volume: 100
Mean Flow Rate(Ml/H): 2.0/3.0/4.0
Self-Control Dosage(Ml/Time): 0.5
Bolus Refill Time: 15
Trademark: CHANGSHENG
Specification: ISO/CE
Origin: China
HS Code: 9018907000
Disposable Infusion Pump
Product Name:Disposable Infusion PumpDisposable Infusion Pump
Product Name:Disposable Infusion Pump