For details, click the button below to request information. Yancheng Rongtai Labware Co.,Ltd , https://www.shtestlab.com
Principle and Application of Single Particle Inductively Coupled Plasma Mass Spectrometry
Introduction
Nanotechnology is a fast-growing emerging field, and its development and prospects have brought many great challenges to scientists and engineers. Nanoparticles are being used in a wide range of materials and products such as coatings (for plastics, glass and fabrics), sunscreens, antibacterial bandages and garments, MRI contrast agents, biomedical element labels and fuel additives. However, the element composition of the nanoparticles, the number of particles, particle size and particle size distribution is also a simultaneous rapid characterization problem. Inorganic nanoparticles, characteristics which best satisfies the above-described technique is inductively coupled plasma mass spectroscopy method (ICP-MS) in a single particle mode. When analyzing single nanoparticles using ICP-MS , it is necessary to use a different method than the measurement of dissolved elements. This paper introduces the theory behind single-particle ICP-MS measurements and compares them by analyzing the dissolved elements to propose differences.
Understanding single particle ICP-MS analysis
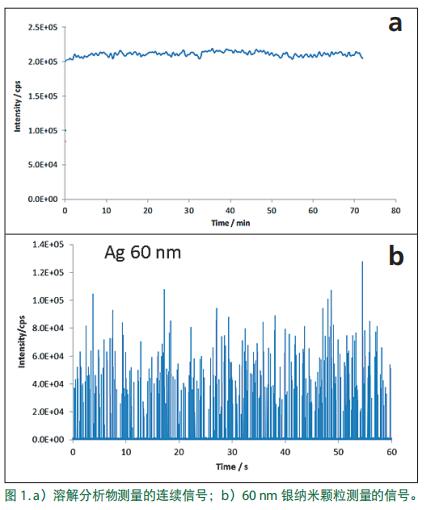
To effectively detect and measure single nanoparticles by ICP-MS , operate the instrument in a different way than when dissolving the sample. The response signals for the dissolved sample and single nanoparticle analysis are shown in Figure 1 . In Figure 1a , the steady state signal is derived from the measurement of dissolved elements; the signal when detecting a single particle is pulsed, as shown by the 60 nm silver particle detection signal in Figure 1b . In Figure 1b , each peak represents a particle. Difference data collection is key to understanding the different single particle analysis, to be understood that this part, the simplest method is to analyze the temporal flow element and measuring the comparative dissolution of the particles involved.
Solubility analysis using ICP-MS
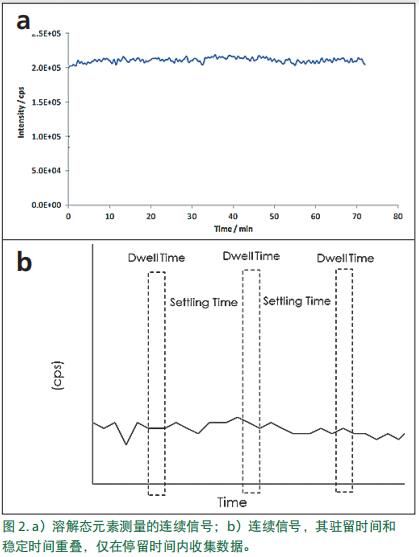
When measuring dissolved elements, the aerosol enters the plasma and the droplets are desolvated and ionized. The generated ions enter the quadrupole and are resolved by their mass -to-charge ratio ( m/z ). In the quadrupole mass to charge ratio (m / z) dwell time, and then moves to the next mass to charge ratio (m / z); each of mass to charge ratio (m / z) analysis time is referred to as "resident time". After the measurement of each dwell time is completed, the stabilization of the electronic device is performed for a certain period of time before the next measurement is performed. This period of time is referred to as "stability time", ie pause and processing time. When analyzing dissolved elements, the resulting signal is essentially a steady state signal, as shown in Figure 2a . However, in consideration of the residence time and settling time, due to the settling time of the electronic device, because of this fact, the detection signal is not continuous, and this is a key point nanoparticle analysis (FIG. 2b).
For dissolved ions, the missing part of the signal is not important because the element dissolves and produces a continuous signal .
Single particle analysis using ICP-MS
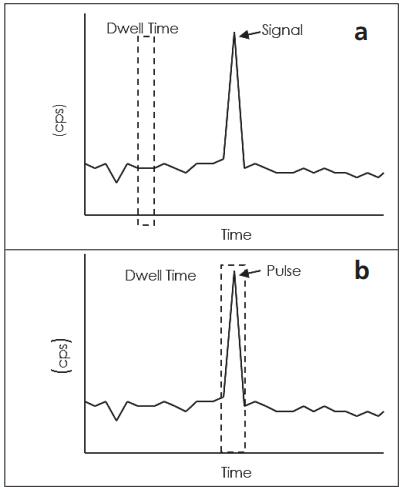
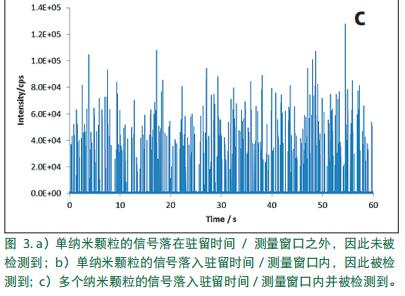
In the same manner as in the dissolved solution, the aqueous solution of the particles into the plasma. When the droplets are desolvated in the plasma, the resulting particles are ionized to produce a large amount of ions (each particle forms an ion cloud). The ions then enter the quadrupole. However, when using a conventional ICP-MS data collection number of ways, and alternately between settling time and residence time, it can not always be detected by the ion cloud. For example, if the ion cloud falls within the dwell time window, it can be detected. Otherwise, if the quadrupole ion cloud enters the detector or to the stability in the time, it can not be detected, resulting in inaccurate count. Those illustrated in Figure 3a, if a single particle ( "signal" peak) ion cloud falling dwell time window may not be detected. As shown in Figure 3b , the ion cloud can be detected when a single particle ion cloud falls within the dwell time window. When rapid succession a plurality of particles detected, the resulting signal is a series of peaks, each peak comes from a particle, specifically shown in Figure 3c.
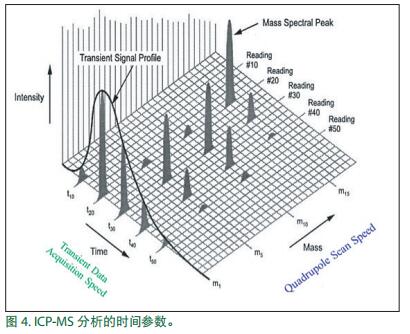
Time parameters of single particle ICP-MS
Figure 4 shows the time parameters involved in the ICP-MS analysis. Three coordinate axes represent the signal intensity, mass to charge ratio (m / z) and time. For conventional / dissolved analysis, mass to charge the highest importance shaft axis and the signal intensity ratio of: is a graph derived spectrum m / z and the signal strength. The time axis is important when considering the moving speed of the quadrupole from the mass-to-charge ratio to the mass-to-charge ratio , and this parameter is called "quadrupole scanning speed". Quadrupole scan speed plays an important role in measuring multiple elements of transient signals, such as laser ablation and multi-element morphological analysis .