Medical Network February 26th, the first domestic Ma Luohua, listed!
On February 25, the State Food and Drug Administration announced that the first domestic biosimilar drug rituximab was approved for marketing.
The State Food and Drug Administration notified that it approved the application for the registration of rituximab injection (trade name: Hanlikon) developed by Shanghai Fuhong Hanlin Bio-Pharmaceutical Co., Ltd. This drug is the first biosimilar drug approved in China and is mainly used for the treatment of non-Hodgkin's lymphoma.
What is a biosimilar?
A biosimilar drug is a therapeutic biological product that is similar in quality, safety, and efficacy to a reference drug that has been approved for registration.
According to the State Food and Drug Administration, China has become the country with the largest number of biosimilar drugs in the research. More than 200 biosimilar drug clinical trial applications have been approved, and some products have completed phase III clinical trials and submitted applications for listing registration.
Rituximab (Rituximab) is the first monoclonal antibody to be used in the treatment of cancer by Genentech and approved by the FDA. The rituximab injection filed by Fuhong Hanlin Company was the first product in China to be developed and declared according to the biosimilar drug route with rituximab as a reference drug. It was the first approved. Domestic merlot.
The State Food and Drug Administration has included the product in the priority review and approval process in accordance with the requirements of the “Opinions on Deepening the Reform of the Examination and Approval System and Encouraging the Innovation of Drug Medical Devices â€, “Supporting the Imitation of Biosimilar Drugs and Medical Device Combination Products with Clinical Valueâ€. At the same time as the technical review, the production site inspection and inspection work was started simultaneously, which speeded up the time-to-market of the product.
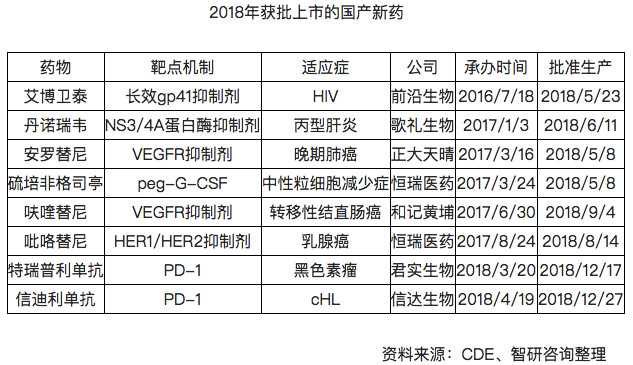
New drug listed in 2018
With the promulgation of the State Council policy, it is required to “accelerate the registration and approval of new anti-cancer drugs at home and abroad to meet the urgent needs of patientsâ€. In recent years, the number of approved new drugs in China has been greatly improved. According to the data of the State Food and Drug Administration, 18 new anti-cancer drugs were approved for listing in 2018.
In 2018, China's biomedical field has achieved breakthrough development, and China's innovative pharmaceutical industry has also begun to break out. According to the statistics of Zhiyan Consulting, 8 heavy domestically produced new drugs have been approved for listing in 2018.
The domestically-produced new drugs have been approved for listing, mainly for two reasons:
On the one hand, it is the promulgation of the “Opinions on Deepening the Reform of the Examination and Approval System and Encouraging the Innovation of Pharmaceutical Medical Devicesâ€, shortening the approval process for new drug clinical trials. In July 2018, the State Food and Drug Administration made adjustments to the review and approval of drug clinical trials: within 60 days from the date of application acceptance and payment, the applicant did not receive any negative or questioned opinions from the Drug Evaluation Center of the State Food and Drug Administration. , can be tested according to the submitted plan;
On the other hand, China's bio-pharmaceutical companies long-term accumulation of technical experience help to improve the efficiency of research and development of new drugs, the number of applications to promote growth of China-made drugs.
The long-term investment in new drug research and development of Chinese pharmaceutical companies has gradually shown good results in recent years. The accelerated increase in the scale of clinical application of new domestic drugs also reflects the enhancement of the research and development strength of new drugs in China. In the domestic pharmaceutical market, the domestic drug market and import substitution The market is expected to have considerable room for growth.
The State Food and Drug Administration said that it will further deepen the reform of the drug review and approval system, continue to intensify its efforts, continue to accelerate the implementation of a series of policy measures for the listing of new drugs overseas, and speed up the registration and approval of new anti-cancer drugs to better meet the clinical needs of patients. .
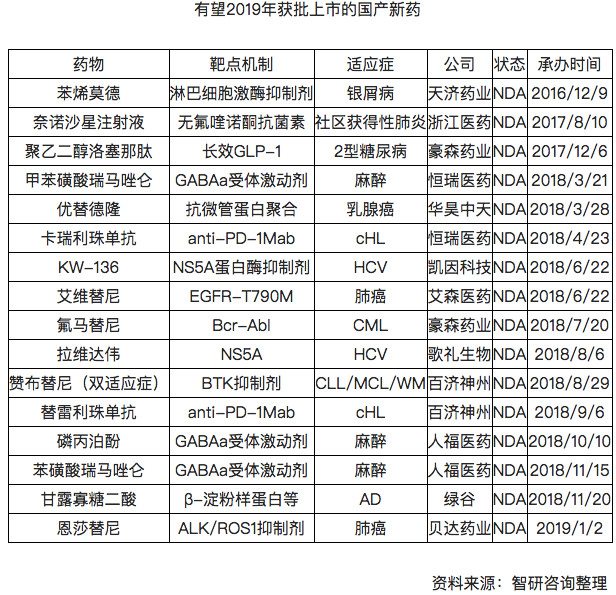
It is foreseeable that the number of approved domestically produced innovative drugs in 2019 may increase further.
Air Purifier
Dynamic disinfection is a process of disinfection and cleaning of air and surfaces in indoor Spaces. A
new method of safe disinfection that does not cause harm or interference to people and the environment on
site.The charged particles and active oxygen components produced by the low-temperature plasma in the
equipment play a major role in the process of killing bacteria and viruses, and have the effect of
broad-spectrum sterilization. It can not only inactivate aerosol plankton in the air and bacteria
and viruses on the surface of object but also effectively decompose formaldehyde and volatile organic
compounds, degrade all kinds of bad smell and purify it Air environment.A small amount of high purity
zone.
Air purifiers, also known as "air cleaners", air purifiers, and purifiers, refer to decoration pollution that can absorb, decompose or convert various air pollutants (generally including PM2.5, dust, pollen, odor, formaldehyde, etc. , bacteria, allergens, etc.), household appliances that effectively improve air cleanliness, are mainly divided into household, commercial, industrial, and building.
There are many different technologies and media in air purifiers that enable it to provide clean and safe air to the user. Commonly used air purification technologies include: adsorption technology, negative (positive) ion technology, catalytic technology, photocatalyst technology, superstructured photomineralization technology, HEPA high-efficiency filtration technology, electrostatic dust collection technology, etc.; material technologies mainly include: photocatalyst, activated carbon, Carbon core filter technology, synthetic fibers, HEAP high-efficiency materials, negative ion generators, etc. Most of the existing air purifiers are composite types, that is, a variety of purification technologies and material media are used at the same time.
Air Sterilizer,Air Disinfector Purifier,Air Cleaner Machine,Air Disinfectant Spray
Jilin Sinoscience Technology Co. LTD , https://www.jlgkscience.com