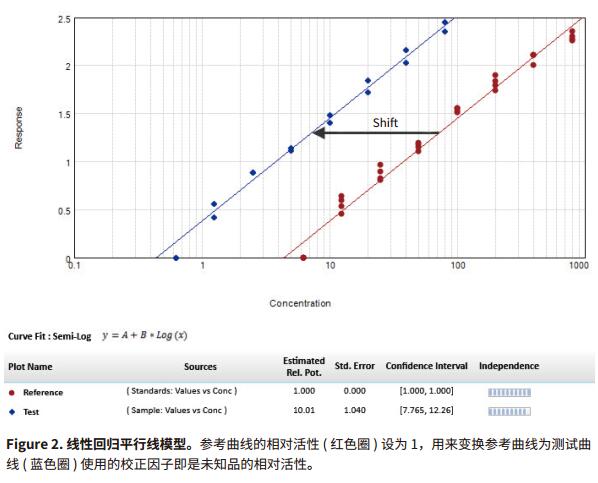
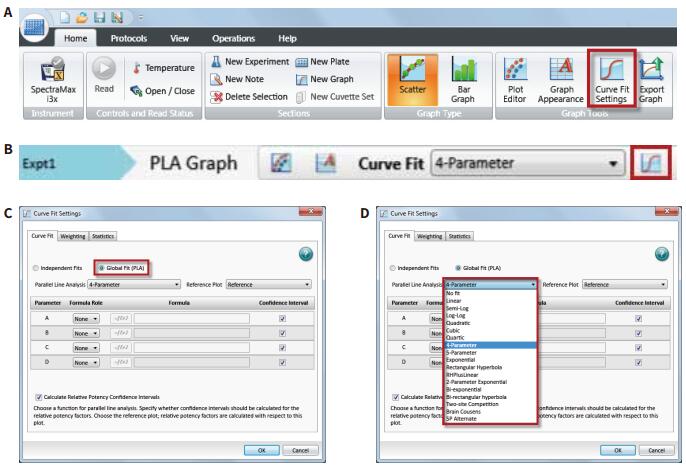
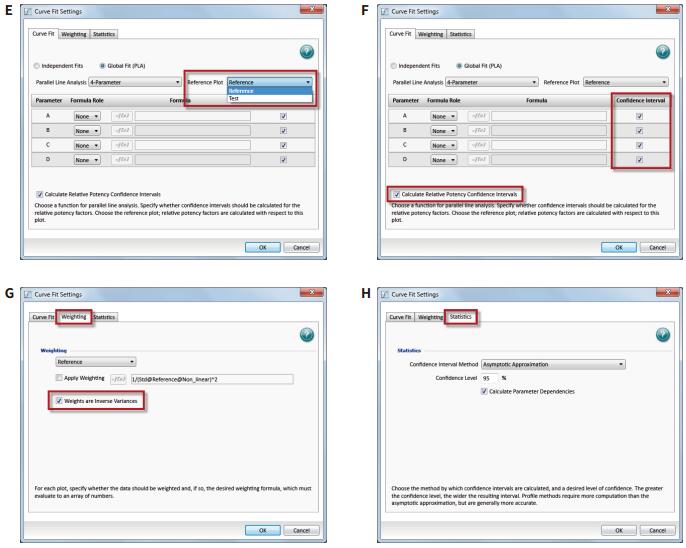
Introduction Parallel line analysis (PLA) methods are often used in biological experiments. When it is not possible to directly detect the activity of a biological product and only by detecting its effect, the PLA method is usually used to compare the effect curves. To get the effect of this product (Figure 1). Parallel line analysis can be used for evaluation if the biological response of the two substances is similar or a similar effect curve is produced for the same substance in two different biological environments. Evaluation of parallelism is a prerequisite for calculating the relative activity of a compound or drug, and is used in many biological applications such as comparison of drug activity, analyte identification, cross-reactivity, interfering substances, matrix compensation, concentration estimation, and inhibition effects. When two curves are judged to be parallel, the function of one of the curves can be obtained by multiplying a scale factor by the function of the other curve to the left or right of the X-axis: f(x)=f(rx), where x Is the dose, r is the proportionality factor or relative activity. For relative activity, the activity of the reference product (known product) is generally set to 1. You can use a scale factor to convert the curve of the reference product into a test article (unknown product) curve, then the proportional coefficient is called test. The relative activity of the product. This method is very suitable for linearly fitted curves because the curve of the linear fit curve is consistent across the entire concentration range (Figure 2). However, for nonlinear fitting curves such as 4-parameter and 5-parameter curves, the S-shaped concentration effect curve has an altered slope value over the entire test concentration range (Figure 1). The methods used to evaluate parallelism can be roughly divided into two categories according to the verification methods of parallelism hypothesis: effect comparison method and parameter comparison method. This article will explain in detail the two types of methods and how to use the SoftMax® Pro 7 software for parallel testing. We have established a protocol that includes an F-test for F-test probability testing and a card-square test for cardinal probability testing. In addition, for the parameter comparison method, according to the Feller's theorem, we also set up a ParallelismTest protocol in the library of SoftMaxProProtocol. All of these methods can be used to evaluate linear and nonlinear fit curves. Advantage - One-click application of universal constrained model fitting Parallelism detection Effect comparison Biological systems often exhibit undesirable behaviors and some noise and fluctuations in the data; therefore, choosing the right curve fitting method and weighting factor to eliminate these noises and fluctuations is the first step before parallel evaluation. If an inappropriate fit is chosen, it is possible to introduce deviations into the parallelism evaluation system and lead to erroneous conclusions. Since it is difficult to obtain a completely parallel fitting curve based on the data in actual experiments, especially for nonlinear fitting regression, it is difficult to calculate the relative activity of the non-parallel curve. In the effect comparison method, the curves of the reference product and the test article are simultaneously fitted using the constraint model, in which the curve is forced to become parallel; in the unconstrained model, the two curves are each fitted. Statistical methods are used to compare the differences in the fit between the constrained model and the unconstrained model, which may be due to non-parallelism. In the constraint model, all the parameters used to describe the curve are the same, except that the parameters used to describe the X-value are different; in a linear fit, the X-value refers to the intercept, and in the nonlinear fit it refers to the upper The median value between the lower asymptotes, that is, the EC 50 value. SoftMax Pro software has the ability to calculate the relative activity of a linear fit, and can also evaluate and calculate the relative activity of a nonlinear fit curve using a constrained model (global fit model) and an independent model. - ChiSquaredPLA (PlotName@GraphName): Returns the card statistic of the fitted curve Note: All of the above formulas are only available if the Global Fit (PLA) option in the Curve Fit option is used. PlotName@ GraphName is the full name of the curve and contains the name of the chart, for example, Plot#1@Graph#1 or Std@Standardcurve. Specifying a curve as a curve within a formula is subjective, and in fact the chi-square value is calculated from all the curve data in the chart. The template named "Parallel Line Analysis Using Ftest and Chi-squared Test" is an SMP template based on these two statistical methods. When the instrument is used to directly acquire data or import data from the outside into the template, all calculations are automatically performed and a null hypothesis is evaluated to determine if the curves are parallel. In this template, the probability values ​​of the F-test and the card-square test must be greater than or equal to 0.95 to determine that the curves are parallel. In general, this setting means that there is a 95% confidence that the assumption is true. The degree of confidence can be adjusted to suit the acceptance level of different experiments. Virtual hypothesis test In the effect comparison method, the calculation of parallelism is usually performed by using the function of residual square sum (RSSE) to judge the fitting effect of the constraint model on the data. Another special method is based on the addition. The analysis of the sum of squares is performed. This statistical regression method is a form of analysis of variance (ANOVA) in which the null hypothesis is that the constrained model is correct or that the curves are parallel to each other. Validation of null hypotheses can use a variety of different statistical techniques, including probabilistic F-tests or chi-square tests. In both methods, the probability value is calculated. Usually this value is between 0-1. When this value is close to 1, it means that the parallelism between the curves is increasing. It should be noted that the F-test has certain defects. This method may produce a false positive judgment for a curve with a high degree of fit to an independent model or a false negative for a curve with a low degree of fit for an independent model. Both methods are included in the SoftMax Pro software. The test value and probability value calculations can be easily obtained by the following formula in the software: Noise and weight Noise is a random change in the detection response, which is an important factor to consider when conducting parallel line evaluations because it affects the ability to detect parallelism. In the case of high noise, the parallel calculation value cannot be correctly reflected in the degree of non-parallelism because it is excessively affected by noise. For both the F-test and the chi-square test, the processing power for different noise levels is different in the process of calculating the respective probability values. The probability value of the F-test is not affected by this factor, but the calculated value of the Kafang test probability is highly dependent on the noise level and requires an accurate estimate of the data variance. Therefore, for the chi-square test, the inverse variance weight setting is required. As discussed in the "Selecting the best weighting factor in SoftMax Pro 7" application note, biological experiments generally have a large variance in the upper half of the curve. In a weightless regression analysis, the results of parallelism are primarily affected by the data points in the upper half of the curve, while the data points in the lower half of the curve have little effect on the parallelism results. In the template of the software, the weighting factor is set using the reciprocal of the variance, but can be adjusted to a more appropriate weighting formula depending on the situation. In addition, in SoftMax Pro 6.5 or higher, the reciprocal weighting of the variance can be set directly (Figure 4G). In the chi-square test method, the confidence interval setting of the parameter (Figure 4H) can only be used if the 'Weights are Inverse Variances' item is selected. The setting of the best weighting factor will ensure that the result is mainly from all the changed data points. How to use PLA in SoftMax Pro In SoftMax Pro 7 software, PLA can be used in all general curve fitting modes except for the “point-to-pointâ€, “log-logit†and “cubic spline†fitting methods. In the chart section, all curves have a common curve fitting equation, and the specific usage settings of the PLA are shown in Figure 4. Parameter comparison Compared with the response comparison method, the difference in response under different dose curves is directly evaluated. The parameter comparison method will independently compare the parameters of each curve under the unconstrained model, and refer to the approximate confidence interval; the parameter pair must fall under the specified confidence level. Within the confidence interval. This method is called the equivalent test and the test method in which the parallelism is less than the specified threshold. The slope ratio method used in the European Pharmacopoeia is an example of an equivalent test method. Feller's theorem The Feller's theorem uses a statistical method to calculate the confidence interval for the ratio of the two parameters, and uses the tInv function to calculate the ratio, thus following the t-distribution with degrees of freedom, with a probability of p. The statistical formula of SoftMaxPro software calculates the confidence interval for the ratio between the reference curve and the curve fitting parameters of the test curve, and establishes a template (Figure 5) containing these calculations and a probability threshold setting of 0.1 (90% confidence). Can be adjusted accordingly. In order to determine whether the reference curve and the test curve are parallel, it is necessary to compare the calculated confidence interval with a fixed confidence interval of a certain confidence level; in this template, a 90% confidence level is used, so if the calculated confidence interval falls on Between 0.9 and 1.1, then these two curves will be considered parallel. For a linear fit curve, the test is performed for the slope values ​​of the reference and test curves, which are the B-parameter values ​​within the curve equations described in the SoftMaxPro software. However, for nonlinear fitting curves, this method only tests the upper platform and slope values, while the lower platform does not test because of the mathematical limitations of the Feller's theorem. At low concentrations, the variation in parameters can be very high, causing the intermediate calculation process to produce imaginary numbers, which in turn leads to incorrect or uncalculated confidence intervals for the final calculation. This issue was suggested by the US Department of Agriculture's (USDA) Center for Veterinary Biologics to fix the lower platform value to zero and then use the upper platform and slope values ​​for testing. Similarly, in the template of Parallelism Test of SoftMax Pro software, the lower platform value is set to 0 by default, and then the parallelity conclusion is judged for the slope (parameter B) and the parameter A or D as the upper platform. in conclusion Numerous biological experiments require the parallelism of two dose-effect curve pairs. This application note describes how to use the effect comparison method of F-test and chi-square test and the parameter comparison method described by Feller's theorem for parallel line analysis. . In SoftMax Pro 7 software, you can choose to use constrained and non-binding curve fitting models as well as advanced statistical formulas to calculate the analysis. At the same time, in the SoftMaxPro7 software, a pre-stored template for each method is provided for the user to call, and the complicated parallel line analysis can be easily performed. References 1. Gottschalk, PD and Dunn, JR 2005. Measuring parallelism, linearity, and relative potency in bioassay and immunoassay data. Journal of Biopharmaceutical Statistics 15(3): 437-463. Canned Baby Corn Whole,Baby Corn Whole Canned,Baby Corn Whole Tin,Corn Whole Canned ZHANGZHOU TAN CO. LTD. , https://www.zztancan.com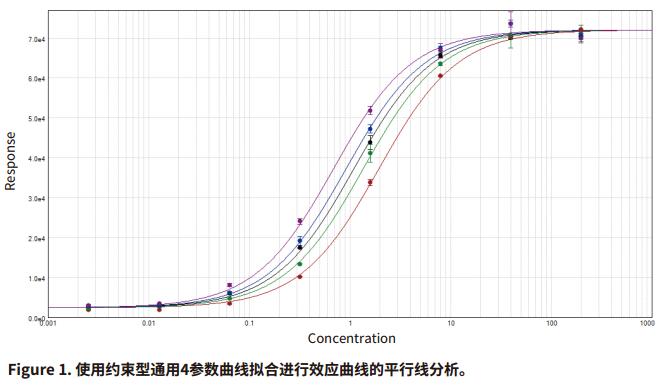
- Automatic calculation of relative activity, fitting parameters and confidence intervals
- Preset various parallel line analysis template calls
- ChiProbabilityPLA (PlotName@GraphSection): Returns the probability distribution value of the card statistic of the fitted curve
- FStatPLA (PlotName@GraphSection): Returns the F-test value of the fitted curve
- FProbPLA (PlotName@GraphSection): return the F-test value probability distribution value of the fitted curve 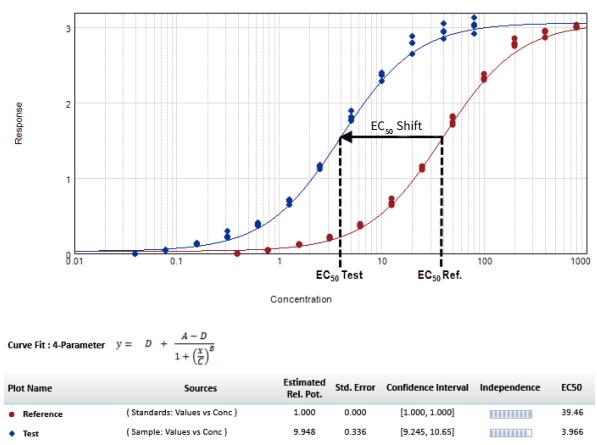



2. Bates DM and Watts DG 1988. NonLinear Regression Analysis and its Applications. New York, Wiley.
3. Draper, NR and Smith H. 1998. Applied Regression Analysis. 3rd Ed. New York, Wiley.
4. Buonaccorsi, JP 2005. Fieller's Theorem.In: Armitage, P., Colton, T., editors. Encyclopedia of Biostatistics. 2. Vol. 3. New York, Wiley.
5. United States Department of Agriculture Center for Veterinary Biologics Standard Operating Policy/Procedure. 2015. Using Software to Estimate Relative Potency. USDA Publication No. CVBSOP0102.03. Ames, IA.