Introduction: How to accelerate the drug development process? What kind of weapon can solve the bottleneck of drug development? How to develop modern new drugs in valuable and abundant natural medicine resources? The proliferation of macromolecules such as monoclonal antibodies, vaccines, and recombinant proteins is exploding. Do you expect to develop new drugs as soon as possible to occupy a place? Molecular Devices offers a complete solution for the most critical steps in the drug development process. Drug screening is the testing and testing of preliminary pharmacological activities of substances that may be used as medicinal substances. For innovative drugs, screening is an indispensable means and approach. In particular, the competition for contemporary innovative drug research is very intense. The focus of competition lies in the screening of new drugs. Low-cost and high-efficiency screening of new drugs is the core of the problem. It is the process of shortening the discovery of new drugs. Molecular Devices is committed to providing innovative solutions for traditional small molecule and macromolecular drug development. Its products are known in the industry for its high throughput, cost saving, high automation, high stability, easy operation and use, and are recognized by the industry as a leader in high-throughput drug screening technology. Ion channels are a class of transmembrane proteins encoded by genes that are located on the cell membrane and are passive transport pathways for various inorganic ions across the membrane. Ion channel structure changes and dysfunction caused by gene mutations or drug effects are related to the occurrence and development of a series of diseases, and are called ion channel diseases. In recent years, the development of drugs targeting ion channels has attracted more and more attention, becoming the third major target for drug screening. On the other hand, the detection of toxic side effects of the hERG channel has become an essential step in the early safety screening of drugs. It has been found that almost all clinical drugs have LQT or TdP applied to hERG, and the drugs that cause hERG inhibition have no obvious commonality in chemical structure, which is difficult to predict and can only be solved by experimental means. In 2004, both ICH and the US FDA issued regulations on non-clinical testing of Ikr (mainly hERG), which requires that the current changes in the ion channel must be provided when the drug is marketed, otherwise the new drug should not be used in the clinic. To this end, new early drug safety assessment methods need to be introduced into the pharmaceutical R&D process in order to detect potential cardiotoxicity of candidate compounds early and minimize the investment and risk of new drug development. I. Fluorescent dye labeling method 1. Calcium sensitive fluorescent dye 2. Voltage sensitive fluorescent dye 2. Patch clamp technique for screening ion channels 1. Screening ion channels using IonWorks Barracuda high throughput patch clamp system 2. Using IonFlux to study ion channels 3 Manual patch clamp system Download the original to learn more nitrile glove,latex glove,PVC glove,single pair packed latex glove,rubber glove Shandong Zhushi Pharmaceutical Group Co.,LTD , https://www.sdzs-medical.com
Ion channel drug development and screening 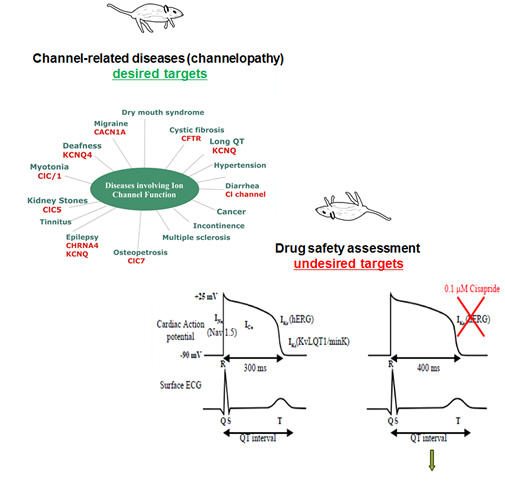
related information:
One of the complete solutions for drug development and screening: GPCRs drug development and screening
Complete Solution for Drug Development and Screening: Kinase and Membrane Transporter Drug Development and Screening
Complete solution for drug development and screening: TCM modernization and high-throughput drug development and screening
Complete Solution for Drug Development and Screening: Monoclonal Antibody Drug Development and Screening