Matthew A. Lauber, Stephan M. Koza and Kenneth J. Fountain Application advantage ■Two hybrid particle columns with unique selectivity (BEH130 C 18 and CSH130 C 18 ). ■When using the optimal concentration of HOAc to adjust the mobile phase, the target peptide peaks obtained by BEH130 C 18 and CSH130 C 18 were narrower and the resolution was higher than when 0.1% TFA was used. With this property, peptides containing medicinal counterions can be obtained in fewer steps. ■BEH130 C 18 and CSH130 C 18 were tested for quality control by trypsin digest of cytochrome c. Waters Solutions Keywords <br>reverse phase (RP), peptide, acetic acid (HOAc), surface charged hybrid particles (CSH), CSH130 C 18 , BEH130 C 18 , preparative chromatography, peptide separation technique (PST) Introduction This article studies the use of BEH C18 The process of preparative peptide separation with a CSHTM C 18 column. BEH C18 It is a silicone C 18 stationary phase based on ethylene bridge hybrid particle (BEH) technology with excellent durability and pH stability. Surface charged hybrid particles (CSH) C 18 is BEH C18 The upgraded product has a surface modified to have a weak positive charge under acidic conditions. The following data demonstrates that both phases are suitable for high sample loading of peptides, both for TFA-regulated mobile phases and for HOAc-adjusted mobile phases. And, at high sample loading, BEH C18 Both the target peaks obtained with CSH C 18 when using the optimized HOAc mobile phase were narrower than when 0.1% TFA was used. Experimental sample description Method conditions (unless otherwise stated) LC Conditions <br>System: ACQUITY UPLC H-ClassBio System with 20-cm Column Compartment Test Conditions: ACQUITY UPLC TUV Detector with 500-nL Analytical Flow Cell A: 0.1% (v/v) HOAc aqueous solution MS Condition <br>Mass Spectrometer: Xevo G2Q-Tof Results and discussion Low purity synthetic peptide Based on this, the mobile phase conditions for the optimal peak shape of CSH130 C 18 and BEH130 C 18 should be different. For this purpose, separation was also carried out using a mobile phase adjusted with 10 times acidic acid (1% HOAc). Although medium concentrations may have some reference value for the development of purification processes, they have not been evaluated here. As shown in Fig. 3, after changing the mobile phase composition, the peak shape of the CSH column is greatly improved, but the peak shape of the BEH column becomes worse. Both BEH columns (0.1% HOAc) and CSH columns (1% HOAc) produced narrower target peptide peaks (half-width widths of 0.5 and 0.6 min, respectively) when using their respective best HOAc mobile phases. For reference, 0.1% TFA was used as the ion pair reagent, as shown on the right side of Figure 3. In use TFA, the target peptide CSH130 C 18 peak width 0.6min, 1.1min compared with the BEH130 C 18. Regardless of which column packing is used, the peptide target peak obtained using the optimized HOAc mobile phase is much narrower than the TFA mobile phase. These results indicate that acetic acid flow is more practical than peptide preparative separation. Above we have only discussed the results of medium preparative loading (1 mg) of synthetic peptides. Figure 5 shows the chromatogram obtained by loading 4 mg of peptide. This loading corresponds to 0.5 g of feedstock, which is a very high single injection productivity for larger 50 mm id columns. It is clear from these data that both CSH and BEH columns are suitable for high sample loading. It is worth noting that from the semi-preparative (50 μg) to the preparative (4 mg), the peak shape obtained by the CSH column remains surprisingly consistent. This high degree of predictability can be very useful when you need to develop separation methods without consuming large amounts of sample. Conclusions <br>5-μm BEH130 C 18 and 5-μm CSH130 C 18 have been shown to be beneficial for the separation of preparative peptides when using mobile phases containing TFA or HOAc, based on the loading studies performed on analytical diametric columns. Great potential. Both the BEH130 C 18 and CSH130 C 18 columns have beneficial properties. Under acidic conditions, CSH130 C 18 exhibits a higher loading capacity compared to BEH130 C 18 and generally produces a narrower target peak. Therefore, the CSH130 C 18 fractional volume is less, and this feature may be helpful for subsequent purification and solvent removal steps. BEH130 C 18 is ideal for preparative separation in neutral/alkaline pH environments due to its longer and higher temperature stability under these conditions. Finally, CSH130 C 18 and BEH130 C 18 each exhibit unique selectivity and are an effective match for solving difficult impurity/target peptide analysis problems. References
Have you ever heard about the Video Surveillance System? The Video Surveillance System is an important part of the Security and Protection Camera field, which has brought us great convenience in all kinds of feilds such as the industry, commerce, education,forest protection, animal and plant research, home secuirty and so on.
The Video Surveillance System is the combination of the Video Capture Device(also called the Camera) and the Video Storage Device(also called the Video Recorder), and we usually call this kinds of combination(the Camera&the Video Recorder)as the CCTV kit/ CCTV Kits/ CCTV Cameras Kit/ CCTV Kits UK/ CCTV Kit for Home/ Boat CCTV Kit.
CCTV kits ,Wireless Nvr Kit,Security Camera Kit,CCTV Camera Kits SHENZHEN SANAN TECHNOLOGY CO.,LTD , https://www.sanan-cctv.com
Waters Corporation (Milford, MA, USA)
ACQUITY UPLC® H-ClassBio System
XSelectTM CSH130 C 18 , 5μm
XBridgeTMBEH130 C 18 , 5μm
MassPREPTM peptide mixture
Peptides have proven to be very useful therapeutic agents and markers in research. Typically, to achieve these uses, the peptide is purified by preparative reverse phase (RP) chromatography. In most cases, the purified peptide must be of high purity. If contaminants are present, they can interfere with the results of the bioanalysis. If contaminants are present in the active pharmaceutical ingredient, the consequences will be severe. Therefore, higher chromatographic resolution is required to minimize co-eluting impurities that are very close to the target peptide chemistry. In addition, column packing is required to have an excellent load capacity to ensure optimum throughput and productivity. The mobile phase used for peptide separation typically contains a strong ion pairing reagent such as trifluoroacetic acid (TFA). However, if a mobile phase containing TFA is used, an additional preparation step is required. Due to the inherent toxicity of trifluoroacetate (TFA salt), it must be removed or replaced 1 . It is best to use a less toxic counterion, such as acetate. In fact, most peptide drugs are acetate or liquid preparations containing acetic acid 2-3 . Therefore, it is more advantageous to replace the TFA mobile phase with a mobile phase of acetic acid (HOAc) as much as possible. Previous studies have shown that after the HOAc mobile phase and isocratic reverse phase chromatography, the peptides in the TFA solution (e.g., crude synthetic peptides) 4-5 are mostly converted to the acetate form 6 . At the same time, a more thorough salt form conversion can be achieved by gradient separation using a high concentration acetate buffer by a simple elution step. 7 In summary, the use of mobile phase HOAc simplify the purification process, advantageous for obtaining the desired peptide and counter-ion in fewer steps.
The MassPREP peptide mixture (part number 186002337, as shown in Table 1) is re-dissolved in 0.1% TFA or 0.1% HOAc (depending on the mobile phase used) so that the total peptide concentration is 0.6 or 2.0 mg/mL (depending on Sample amount). The low purity (<70%) preparation sample DFVGYGVKDFVGVGVK of the synthetic peptide was reconstituted in 0.1% TFA/0.1% HOAc to a concentration of 1 or 4 mg/mL.
Xevo® G2 vQ-TofTM mass spectrometer wavelength: 214 and 250 nm
Scan rate: 2 Hz (filter time constant, 1 s)
Column: XBridge BEH130 C 18 4.6×100mm, 5μm, porous, 130Å (part number 186003579)
XSelect CSH130 C184.6×100mm, 5μm, porous, 130Å (part number 186007077)
Column temperature: 40 ° C
Sample temperature: 10 ° C
Injection volume: 50 to 1000 μL, the loading rate is shown below: 1 mL/min (split after UV detector, injected into the MS source at approximately 20 μL/min)
Mobile phase: see Gradient table vial: LCGC certified clear glass 12 x 32mm screw Qsert vial (part number 186001126C) Gradient: MassPREP peptide mixture
A: 0.1% (v/v) TFA aqueous solution
B: 0.1% (v/v) TFA
90:10 acetonitrile (ACN) / aqueous solution or
A: 0.1% (v/v) HOAc aqueous solution
B: 0.1% (v/v) HOAc
90:10 ACN/water solution Focus gradient of DFVGYGVKDFVGVGVK
A: 0.1% (v/v) TFA aqueous solution
B: 0.1% (v/v) TFA 90:10 ACN/water solution Time (min) %A %B 0 99.5 0.5 1 99.5 0.5 61 40.0 60.0 62 10.0 90.0 65 10.0 90.0 66 99.5 0.5 85 99.5 0.5 Time (min) %A %B 0 90 10 3 90 10 4 80 20 24.2 60 40 29.2 10 90 32.2 10 90 33.2 90 10 52 90 10
B: 0.1% (v/v) HOAc in a 90:10 ACN/water solution or
A: 99:1 (v/v) water / HOAc - 1% HOAc
B:90:9:1 (v/v) ACN/water/HOAc–1% HOAc Time (min) %A %B 0.0 90 10 3.0 90 10 3.3 87 13 23.5 67 33 29.2 10 90 32.2 10 90 33.2 90 10 52.0 90 10
Ionization mode: ESI+
Analyzer mode: Resolution capillary voltage: 3.00kV
Cone hole voltage: 25V
Source temperature: 120 ° C
Desolvent gas temperature: 350 ° C
Cone gas flow rate: 0.0L/h
Desolvent gas flow rate: 800L/h
Correction: NaI, 1 μg/μL, 50 to 2000 m/z
Collection: 50 to 1990m/z,
2Hz scan rate
Data management
MassLynx software version 4.1
Load Study of Peptide Mixtures Containing Nine Components <br>In previous studies, the use of CSH130 C 18 and BEH130 C 18 in the separation of analytical peptides (eg peptide mapping) has been extensively explored 8 -9 . In short, CSH130 C 18 and its innovative surface charging technology improve peak shape and loading compared to other peptide-separated reversed-phase packings. In analytical applications, especially when the mobile phase contains little or no ion-pairing reagent, a significant increase in peak capacity can be observed, up to 90%. Compared to BEH130 C 18 , the positive surface charge of CSH130 C 18 also provides unique selectivity and low retention, making them an excellent match for peptide separation chromatography media.
To investigate the performance of CSH130 C 18 and BEH130 C 18 in preparative separation, a series of different peptides were studied for loading using mobile phases commonly used in production (ie, mobile phases containing TFA or HOAc). A 5 μm particle-filled analytical column (4.6 mm id) was used in these method development experiments.
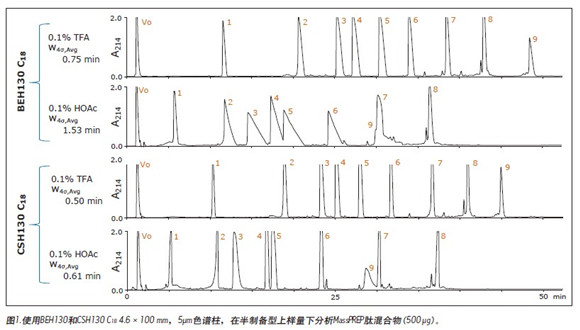
First tested on these columns was the MassPREP peptide mixture, which contained nine different peptides, as shown in Table 1. Figure 1 shows a set of chromatograms of the mixture in a semi-preparative format using BEH130 C 18 and CSH130 C 18 , and two different mobile phases, one containing 0.1% TFA and the other containing 0.1% HOAc. The BEH column had an average 4 peak width of 0.8 min when 0.1% TFA was used. With 0.1% HOAc, the average peak width almost doubled to 1.5 min. The acidity of HOAc is much weaker than that of TFA, resulting in a weakening of the mobile phase acidity and a significant decrease in ionic strength and ion pairing ability. Therefore, it is expected that most of the C 18 columns will have a poor peak shape when using HOAc instead of TFA. This assumption is true for semi-preparative loading of BEH130 C 18 , as shown in Figure 1. However, for the CSH130 C 18 column, the peak shape remained good when TFA was replaced with HOAc. When using a 0.1% TFA mobile phase and a 0.1% HOAc mobile phase, the average 4 peak widths observed in the CSH130 column were 0.5 and 0.6 min, respectively. Peak width data is summarized in Figure 2, where the peak widths of each peptide in the mixture are plotted under different column types and mobile phase conditions, respectively. In addition to the data for the semi-prepared sample loading, the data for the analytical sample loading is also shown. This figure shows that BEH130 C 18 and CSH130 C 18 will produce similar peptide peak shapes under some conditions, including analytical loading of 0.1% TFA. However, under other conditions, such as a semi-preparative loading of 0.1% HOAc, the peak generated by CSH130 C 18 is much narrower. As previously demonstrated by the Institute, 8-9 , CSH130 C 18 obtained a significantly better peak shape of the peptide when using an acidic mobile phase with little or no ion pairing agent. It also shows that this phenomenon is more pronounced when the loading is 20 times higher than the conventional analysis. 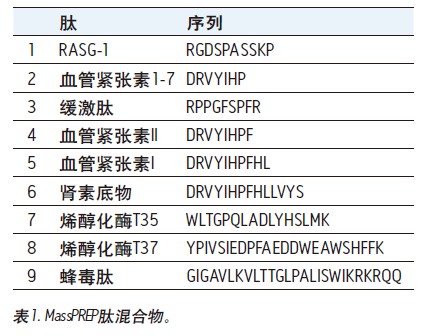
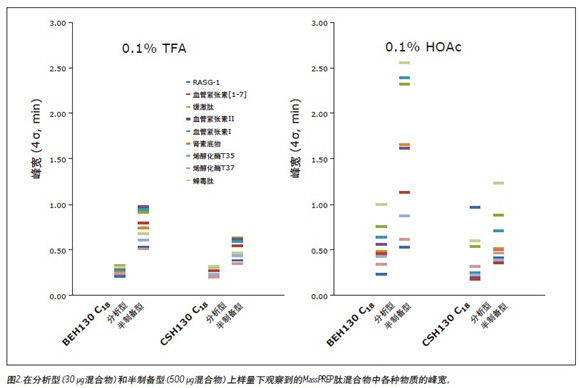
Preparative separation often requires that the amount of sample must be (and sometimes higher than) 1000 times the amount of sample used for analytical separation. A low purity synthetic peptide (a neutral peptide, pI = 6, 1.7 kDa) having the sequence DFVGYGVKDFVGVGVK was used, and the amount of loading below this range and within this range was investigated. Focused gradient separation was used on BEH and CSH columns to reduce run time and low sensitivity wavelength (250 nm) detection to obtain complete peak shape.
The flow was first analyzed using a 0.1% HOAc adjusted flow versus semi-preparative and preparative loading, as shown in Figure 3. The semi-preparative loading on the BEH column (50 μg) produced a significant peptide tail with a significant tailing, which is consistent with the common Langmuirian isotherm. In contrast, at the preparative loading (1 mg), the elution peak of the target peptide was slightly extended, just like the typical anti-Langmuirian isotherm. We know that the anti-Langmuirian isotherm 10 occurs when the peptide is present as a zwitterion. From this, it is known that the mobile phase adjusted by 0.1% HOAc is not sufficiently acidic to fully protonate the carboxyl group of this synthetic peptide, and the peptide may exist as both a cation and a zwitterion. The relative content of zwitterions should increase with increasing sample loading, especially if the target peptide concentration exceeds the protonation/buffering capacity of the mobile phase. This explains the dramatic change in peak shape as the sample loading on the BEH column increases.
Interestingly, as seen in Figure 3, for BEH130 C18 , the use of 0.1% HOAc at preparative loadings appeared to make it easier to obtain narrower target peptide peaks. Under these conditions, the BEH column yields a narrower target peptide peak than the CSH column. In fact, CSH130 C 18 achieved a forward peak at both loadings using 0.1% HOAc. This situation is expected to occur 8-9 because the positive charge on the surface minimizes the tailing of the peptide. Therefore, the forward peak shape is also more obvious. Since there is no tailing peak, the target peak width of the CSH column under preparative loading is actually larger than that of the BEH column. 
Narrower target peptide peaks are often accompanied by higher impurity resolution, giving them the opportunity to collect higher purity fractions. The effect of column packing and mobile phase additives on the DFVGYGVKDFVGVGVK preparative sample loading is shown in Figure 4, with an emphasis on the baseline and the ability of each separation method to separate impurities from the target peak, which have been confirmed by MS. As mentioned earlier, the target peak obtained by the HOAc mobile phase is narrower. Figure 4 also shows that the use of the HOAc mobile phase also minimizes co-elution of the monitored impurities. In addition, it is apparent that the chromatographic selectivity between the target peptide and impurities has changed dramatically by using different mobile phase additives and two different column packings. For the parameters screened by this load study, the CSH column with a 1% HOAc mobile phase provided the narrowest target peptide peak and the least co-elution of the monitored impurities. However, a comparable separation can be obtained using a BEH column and a 0.1% HOAc mobile phase. Column packing with different selectivity and optimum additive concentrations will facilitate the development of highly difficult preparative separation processes. 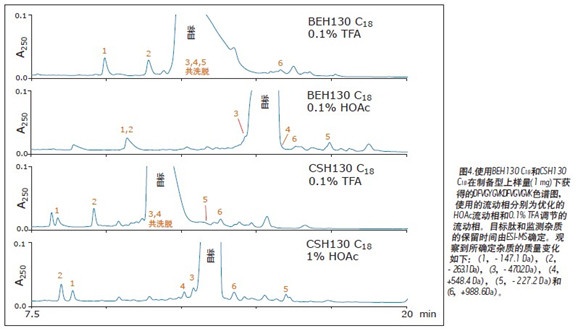
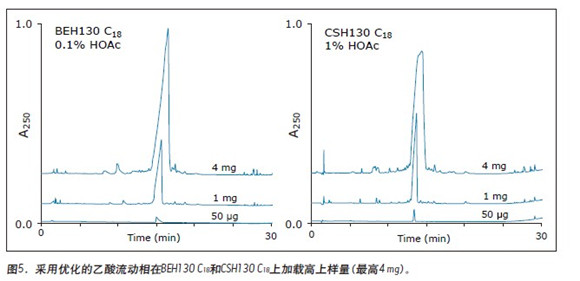
More striking than the above characteristics, the two stationary phases have the best effect on the preparation of the sample-loaded synthetic peptide under different concentrations of mobile phase additives. The best peak shape obtained with optimized HOAc is better than using 0.1% TFA. This means that these hybrid particle C18 columns can be used to simplify the purification process, as peptides containing pharmaceutically acceptable counterions can be obtained in fewer steps using the HOAc mobile phase.
1. Cornish J, Callon KE, Lin CQ, Xiao CL, Mulvey TB, Cooper GJ, Reid IR. Trifluoroacetate, a contaminant in purified proteins, inhibits proliferation of osteoblasts and chondrocytes. Am J Physiol. 1999; 277 (5 Pt 1) : E779-83.
2. Pini A, Lozzi L, Bernini A, Brunetti J, Falciani C, Scali S, Bindi S, Di Maggio T, Rossolini GM, Niccolai N, Bracci L. Efficacy and toxicity of the antimicrobial peptide M33 produced with different counter-ions Amino Acids. 2012; 43(1): 467-73.
3. Reichert JP, Tartat A, Dunn MK. Development trends for peptide therapeutics: A comprehensive quantitative analysis of peptide therapeutics in clinical development. Peptide Therapeutics Foundation. 2010.
4. Fields GB, Carr SA, Marshak DR, Smith AJ, Stults JT, Williams LC, Williams KR, Young JD. In Techniques in Protein Chemistry IV. Hogue-Angeletti R, Ed. San Diego, 1993; 229-237.
5. Kent, SBH. Chemical Synthesis of Peptides and Proteins. Ann. Rev. Biochem. 1988; (57): 957-989.
6. Roux S, Zekri E, Rousseau B, Paternostre M, Cintrat JC, Fay N. Elimination and exchange of trifluoroacetate counter-ion from regulating peptides: a critical evaluation of different approaches. J Pept Sci. 2008; 14 (3): 354-9.
7. Alon H, Butilca GM, Eidelman C, Elster S, Ivchenko A, Shusman S, Tovi A, Zaoui GA. Counterion Exchange Process for Peptides. Patent Pending, 2006; 2010.
8. Lauber MA, Koza SM, Fountain KJ. Peptide Mapping and Small Protein Separations with Charged Surface Hybrid (CSH) C18 and TFA-Free Mobile Phases. Waters Application Note 720004571en. 2013 January.
9. Lauber MA, Koza SM, Fountain KJ. Increasing Peak Capacity in Reversed-Phase Peptide Separations with Charged Surface Hybrid (CSH) C18 Columns. Waters Application Note 720004568en. 2013 January.
10. Gritti F, Guiochon G. Adsorption behavior of the three species of the biprotic peptide Phe-Ala onto an end-capped C18-bonded organic/inorganic hybrid stationary phase. Anal Chem. 2009; 81(24): 9871-84.
So what are the advantages of the CCTV Kit?
Some people may think it more easier and can save much time and energy if they don't need to combine the Camera and the Video Recorder by themselves, which can also help avoiding some errors for those people who don't know much about this Video Surveillance System but need this kind of products to solve some security problems.
How about the disadvantages of the CCTV kit?
The CCTV kit combination is not so flexible as the what some people need. They may prefer to dividually purchase the Camera and the Video Recorder rather that directly choice the CCTV kit that has be combined by the supplier. To dividually choose the models and parameters of these devices and combine them the way they need, then they can get their own CCTV kit, which can more efficiently solve their actual problems.
What kind of people is the CCTV Kit more suitable for?
The CCTV Kit is more suitable for the people who don't know much about the Video Surveillance System and don't know how to combine them. As for the people who have well handled the Video Surveillance System and can precisly know the parameters and functions of the CCTV Camera and video recorder which can perfectly meet their demand, it would be better for them to combine their own CCTV Kit instead of directly chooing the combined CCTV Kit from the supplier.
