Silicone Cleanser Brush,Cleansing Silicone Brush,Best Silicone Cleansing Brush,Silicone Facial Cleansing Brush Shenzhen Jie Zhong Lian Investment Co., Ltd. , https://www.szmeizonscares.com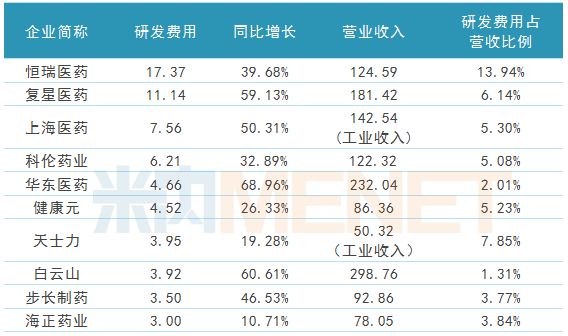

TOP10 of research and development expenses for listed pharmaceutical companies in the first three quarters of 2018
Pharmaceutical Network November 8th, with the disclosure of the third quarterly report of the A-share listed pharmaceutical companies in 2018, the research and development expenses of pharmaceutical companies have also received much attention. According to the statistics of the three quarterly reports of 224 pharmaceutical manufacturing companies disclosed by Eastern Fortune.com, the top ten companies in the first three quarters of 2018 have R&D expenses of more than 300 million yuan, of which 2 companies have more than 1 billion R&D expenses. Yuan, "R&D a brother" Hengrui Medicine led the way with 1.737 billion yuan. High investment, high output, Hengrui Medicine, Fosun Pharma, and Kelun Pharmaceutical's three R&D giants have achieved fruitful results in new drug research and development and consistency evaluation.
Table 1: TOP10 (unit: 100 million yuan) for research and development expenses in the first three quarters of 2018
(Source: listed company's third quarterly report)
Hengrui Medicine: Heavy innovation drugs enter the harvest period
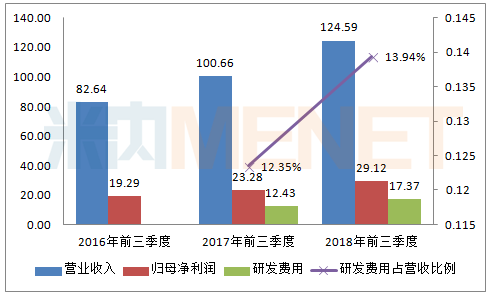
Figure 1: Investment in R&D of Hengrui Medicine in the same period of 2016-2018 (Unit: 100 million yuan)
(Source: listed company's third quarterly report)
According to the data of Hengrui Medicine's third quarterly report, the company's research and development expenses in the first three quarters of 2018 reached 1.737 billion yuan, an increase of 39.68% over the same period of last year. The proportion of research and development expenses to operating income was as high as 13.94%, an increase of 1.59 percentage points over the same period of last year. The R&D expenses in the first three quarters of 2018 directly affected the company's R&D expenses for the whole year of 2017 (1.759 billion yuan). For the increase in research and development expenses, the company indicated that it was due to the increase in research and development investment during the reporting period.
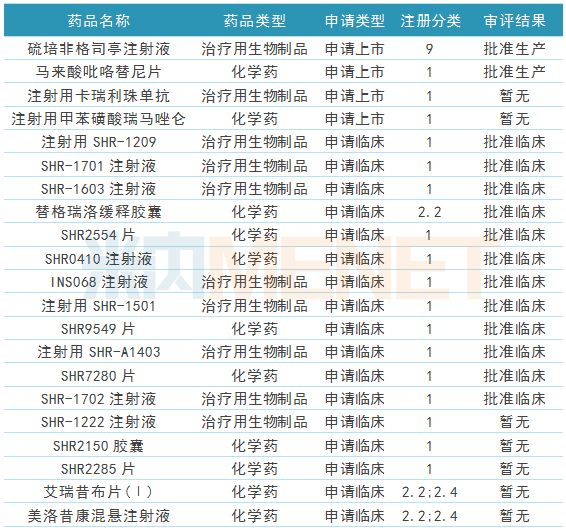
Table 2: Research and development of new drugs for Hengrui Medicine during the reporting period
(Source: Mnet China MED China Drug Evaluation Database 2.0)
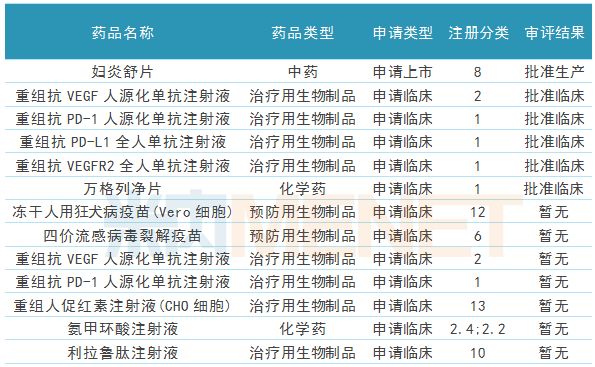
During the reporting period, paragraph 2 heavy HRH medicine approved new drug production, respectively, for the treatment of cancer chemotherapy related neutropenia neutral sulfur culture filgrastim injection (19K), and for treatment of breast cancer Small molecule new drug pyrrolidine maleate tablets. Two types of new drugs were applied for marketing, including rimazolam tosylate for intravenous anesthesia and carelibizumab (PD-1) for injection for the treatment of liver cancer, stomach cancer, lung cancer and other diseases .
In addition, the company has 5 new drugs for clinical application, of which 3 are Class 1 new drugs; 12 new drugs have been approved for clinical trials, 11 of which are Class 1 new drugs, and many products are not listed on the international market at the same time. Foreign giants are also in clinical trials.
Table 3: Progress of Hengrui Medicine's Conformity Evaluation during the Reporting Period
(Source: Mnet China MED China Drug Evaluation Database 2.0)
During the reporting period, four products of Hengrui Medicine passed or agreed to pass the consistency evaluation. The tamsulosin hydrochloride sustained-release capsules and ambroxol hydrochloride tablets were submitted by the company according to the consistency evaluation application procedure. Sexual evaluation; paclitaxel for injection (albumin-bound) and desflurane for inhalation were reported in the old six categories of registration, but because they were included in the "China Listed Drug List", it was deemed to have passed the consistency evaluation.
In addition, the company has 8 generic drug applications for listing. These generic drugs are applied for in the new 3/4 category of registration. After approval, they are deemed to have passed the consistency evaluation; 14 generic drugs are in the supplementary application supplementary stage. After approval, it passed the consistency evaluation.
Fosun Pharma : R&D high investment drags down performance
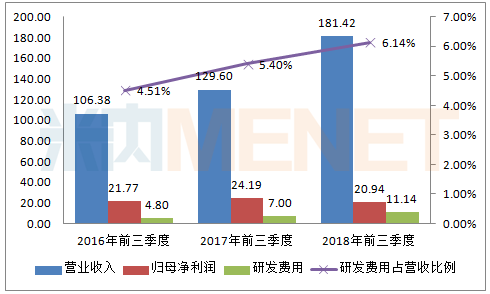
Figure 2: Performance and R&D expenses of Fosun Pharma in the same period of 2016-2018 (Unit: 100 million yuan)
(Source: listed company's third quarterly report)
According to the data of Fosun Pharma's third quarterly report, in the past three years, the company's operating income has increased year by year, but the net profit of the returning mother has declined in the third quarter of 2018. The company believes that the investment in innovation and research and business layout is increasing, and some participating companies lose money and interest. Factors such as increased costs. Fosun Pharma is currently in the period of concentrated investment in research and development, and huge investment in research and development will inevitably drag down profits.
The research and development expenses of Fosun Pharma increased year by year, and the proportion of research and development expenses to operating income also increased year by year. In the first three quarters of 2018, the company's development expenditure was 1.77 billion yuan, of which R&D expenses were as high as 1.114 billion yuan, an increase of 59.13% over the same period of last year. The proportion of research and development expenses to operating income reached 6.14%, an increase of 0.74 percentage points over the same period of last year. The research and development expenses for the first three quarters of 2018 have exceeded the company's 2017 R&D expenses ($1,027 million). According to the company, the change in research and development expenses was mainly due to the continuous increase in the concentrated investment in the research and development investment and consistency evaluation of biosimilar drugs and bio-innovative drugs during the reporting period.
Table 4: Research and development of new drugs for Fosun Pharma in the report period
(Source: Mnet China MED China Drug Evaluation Database 2.0)
As of the end of the reporting period, the company has 9 monoclonal antibody products (4 bio-innovative drugs), 13 indications approved in the country, 2 monoclonal antibody products, 1 combined therapy in China for clinical trial applications. Trastuzumab, adalimumab and bevacizumab in biosimilar drugs, recombinant human mouse chimeric anti-CD20 monoclonal antibody injections of bio-innovative drugs have entered phase III clinical trials, and are expected to submit applications for listing one after another. Rituxumab is declared for production and is included in the priority review and is expected to be approved for listing by the end of 2018.
In the first three quarters of 2018, the company's traditional Chinese medicine Fuyanshu tablets were approved for production, four bio-innovative drugs were approved for clinical treatment, and one class of new drug Wangeleli tablets was approved for clinical treatment; seven new drugs were applied for clinical, and two bio-innovative drugs were applied for. The matter is a new indication.
Table 5: Progress in the evaluation of Fosun Pharma's consistency during the reporting period
(Source: Mnet China MED China Drug Evaluation Database 2.0)
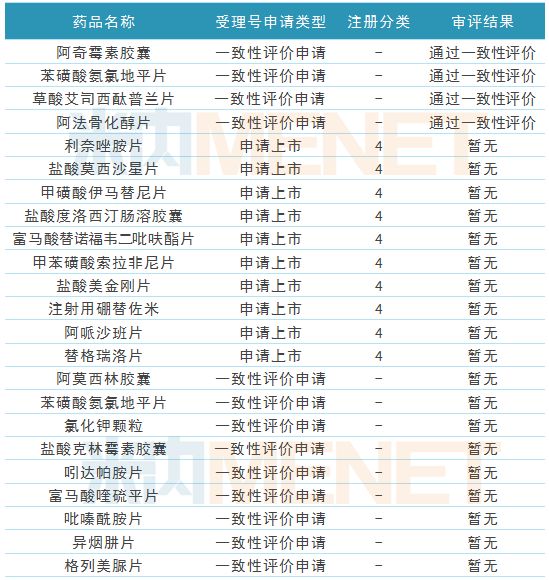
During the reporting period, Fosun Pharma's four products passed the consistency evaluation. Azithromycin capsules, amlodipine besylate tablets and Alfacalcitol tablets were submitted by the company according to the consistency evaluation application procedure. Pass the consistency evaluation. Azithromycin capsules and Alfacalcitol tablets are currently not in the application stage for consistency evaluation.
In addition, the company has 10 generic drug applications for listing. These generic drugs are applied for the new 4 categories of registration classifications. After approval, they will be deemed to have passed the consistency evaluation; 9 generic drugs are in the supplementary application supplementary stage. After the batch, the consistency evaluation was passed.
Kelun Pharmaceutical: R & D and innovative transmission power
Figure 3: Kelun Pharmaceutical's performance and R&D expenses in the same period of 2016-2018 (Unit: 100 million yuan)
(Source: listed company's third quarterly report)
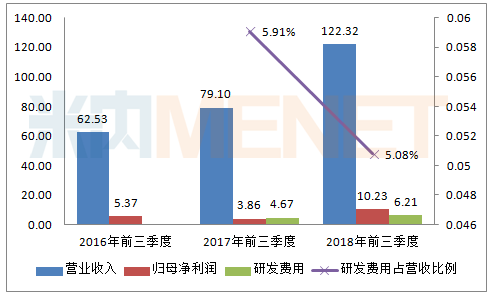
According to the data of the third quarterly report of Kelun Pharmaceutical, compared with the same period of 2016 and 2017, the company's performance in the first three quarters of 2018 was outstanding, and the operating income exceeded 10 billion for the first time, up 54.63% over the same period of last year; the net profit of the mother exceeded 10 for the first time. 100 million yuan, an increase of 164.80% over the same period last year.
In the first three quarters of 2018, the company's research and development expenses reached 621 million yuan, an increase of 32.89% over the same period of last year. The research and development expenses accounted for 5.08% of the operating income. Due to the substantial increase in operating income, the ratio decreased compared with the same period of last year. For the increase in research and development expenses, the company stated that it has vigorously promoted the “innovation-driven†strategy and increased R&D investment during the reporting period.
  Table 6: Research and development of new drugs in Kelun Pharmaceutical during the reporting period
(Source: Mnet China MED China Drug Evaluation Database 2.0)
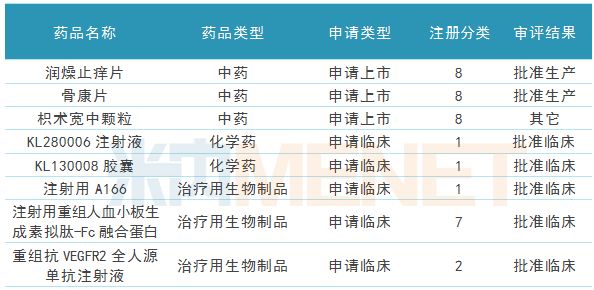
R&D and innovation, as the third engine of Kelun Pharmaceutical, began to power the company's development. During the reporting period, the company had 2 Chinese medicines, 8 generic drugs approved for production, and 1 Chinese medicine application for listing. Five new drugs were approved for clinical trials, three of which were class 1 new drugs.
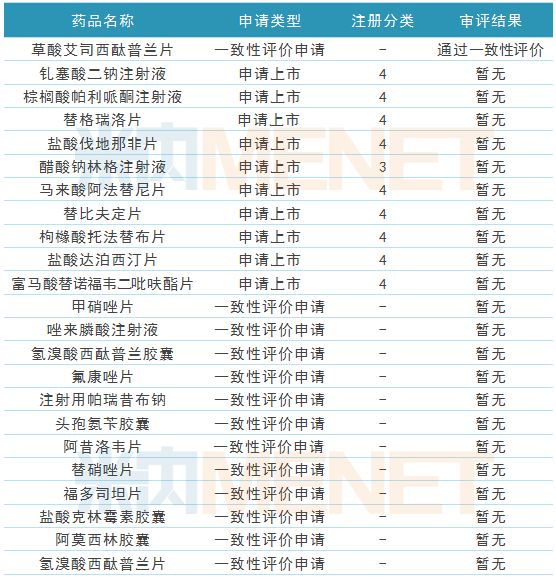
Table 7: Progress of Cologne Pharmaceuticals Coherence Evaluation during the Reporting Period
(Source: Mnet China MED China Drug Evaluation Database 2.0)
During the reporting period, one of the first products of Kelun Pharmaceutical passed the consistency evaluation, and 10 generic drugs were applied for listing. These generic drugs were applied for the new 3/4 registration classification, and they were deemed to be consistent after approval. Evaluation; 12 generic drugs were in the supplementary application stage of the consistency evaluation, and passed the consistency evaluation after approval.
Source: Minenet database, listed company's third quarterly report
Note: Data statistics as of November 6, 2018, if there are any omissions, please correct me