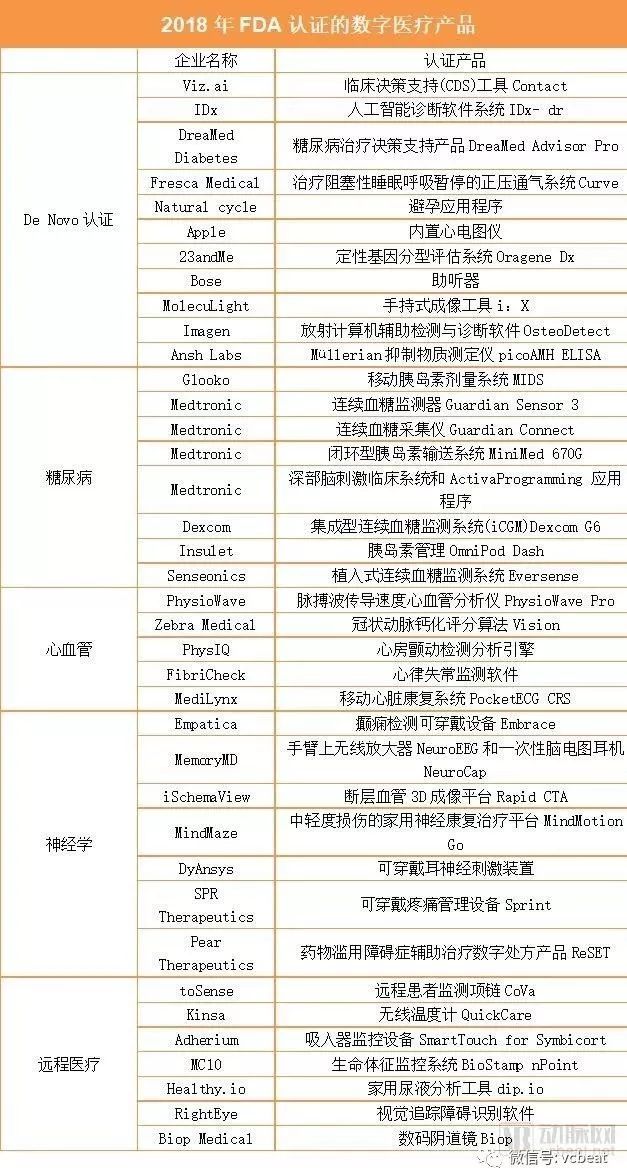
Innovative design, intelligent R&D, and clinical validation are all key steps in launching a medical product , but without the certification of a regulatory agency, many innovative products will never have the opportunity to target patients and consumers. In recent years, the rapid development of digital medical care has spawned many related products. In the United States, the FDA (Food and Drug Administration), which oversees regulation, is constantly introducing new programs and reforming existing regulations to simplify the process of approving new products and to develop the digital healthcare market. The arterial network combs the FDA's regulatory history of digital medical products. The main contents of this article include: 1. Inventory: FDA-certified digital medical products from 2016-2018 2. Review: How does the FDA regulate digital healthcare technologies and products? Inventory: FDA-certified digital medical products from 2016-2018 To date, 41 digital medical products have received FDA certification in 2018. The first major category: De Novo certification (automation class III designation assessment) In February 1.2018, the FDA announced that Viz was allowed. Ai 's Contact is listed. Viz. Ai 's Contact is a clinical decision support (CDS) tool for analyzing CT findings and determining if a patient has had a stroke. In April 2008, the FDA approved the De Novo application for the diagnostic company IDx, allowing its artificial intelligence-based software system IDx- dr to enter the market for automatic detection of diabetic retinopathy in adult patients. This decision marks the first time the FDA has approved the launch of an artificial intelligence-based diagnostic system in the United States that provides test results without the need for a clinician's diagnosis. 3. The DiamMed Advisor Pro, a diabetes treatment decision support product launched by Israeli company DreamMed Diabetes, also received FDA's De Novo certification. The cloud-based DreamMed Advisor Pro is an artificial intelligence software that helps patients manage type 1 diabetes by analyzing data from continuous blood glucose monitors, insulin pumps, and self-monitoring devices to determine the amount of insulin delivered. 4. The connected medical company Fresca Medical has received FDA's De Novo certification to sell its device called Curve, a positive pressure ventilation system for the treatment of obstructive sleep apnea. The Curve includes a flow generator, a lightweight ergonomic air delivery hose and a nasal pillow. Unlike other similar products, the device uses SmartValve technology, which uses less airflow than other systems to help patients with sleep apnea syndrome become more compliant. 5. Natural Cycle's contraceptive app is approved by the FDA to sell this product to pre-menopausal women over the age of 18. This algorithm-driven app was previously approved in the UK and is currently being reviewed by multiple regulators. It helps users track the menstrual cycle by measuring body temperature in the morning and submitting information about the menstrual cycle and telling them when they are most likely to conceive. 6. Apple built an electrocardiograph in the fourth-generation Apple Watch, which has been certified by the FDA. It takes about 30 seconds for the user to do an electrocardiogram and then store the ECG in Apple's health app. With Apple Health Records, some users can also send data directly to their doctor. In addition, the FDA approved an Apple algorithm for detecting atrial fibrillation. In addition to the ECG, it also allows the watch to scan the user's heart rhythm in the background. If the heart rhythm is found to be abnormal, it will send a notification. 7. The 23andMe Personal Genome Service (PGS) is a qualitative genotyping system for genomic DNA isolated from human saliva collected using Oragene Dx OGD 500.001 for simultaneous detection, reporting and interpretation in a wide range of multi-gene assays. Genetic Variation. The assessment system is designed to give users direct access to genetic information that helps to discuss with healthcare professionals. 8. Bose hearing aids are designed to provide sound for patients with mild to moderate hearing impairments 18 years of age or older. Users can make their own adjustments to Bose hearing aids, but without pre-programming or hearing tests. The FDA classifies Bose hearing aids as Class II medical devices under adaptive air-conducting hearing aids for direct sale and use without the assistance of hearing health professionals. 9. MolecuLight i: X is a handheld imaging tool that allows clinicians to diagnose and treat skin wounds and is intended for prescription use only. According to FDA requirements, MolecuLight i:X can be used to view and digitally record wound images, observe and digitally record fluorescent images of the wound as it is exposed to the laser environment. 10. OsteoDetect uses machine learning techniques to analyze wrist X-rays to identify and highlight the distal fracture of the adult wrist and wrist in the anterior (PA) and lateral (LAT) X-ray films. The FDA considers OsteoDetect to be a computer-aided detection and diagnostic software for radiology and classifies it as a Class II medical device. 11. picoAMH ELISA is used for in vitro enzyme-linked immunosorbent assay (ELISA) to quantify the concentration of anti-emergence and esophageal hormone (AMH, also known as Müllerian inhibitory substance (MIS)) in human serum. The aforementioned substances can be used for the menopausal state of women aged 42 to 62 years. The FDA requires that the picoAMH ELISA should be used in conjunction with other clinical and laboratory findings and the results of the test and should not be used alone to make a diagnosis or treatment decision. In addition, the FDA stipulates that the picoAMH ELISA can only be used for in vitro diagnostic use. The second major category: diabetes 12. The Diabetes Digital Health Company Glooko's Mobile Insulin Dose System (MIDS) is FDA-approved and is an application-driven tool that adjusts insulin dose directly from data collected from patient blood glucose meters. MIDS allows clinicians to create a customized treatment plan and send it to patients with type 2 diabetes through the app. 13Medtronic received four FDA approvals in 2018, the first to increase the functionality of the Guardian Sensor 3, allowing patients to wear the sensor on the upper arm. The sensor is part of the MiniMed 670G system and is the only FDA-approved continuous blood glucose monitor that controls the automatic delivery of insulin through a hybrid closed loop system. 14. The second certified product is Guardian Connect. This product can be connected to a smartphone for patients who inject insulin multiple times a day. The Guardian Connect system consists of a Guardian Sensor 3 and an accessory transmitter that continuously transmits blood glucose data to the user's mobile phone via Bluetooth. The application can alert the patient 60 minutes before hyperglycemia or hypoglycemia occurs. 15. The third time is to allow the MiniMed 670G to be opened to children aged 7 to 14 years with type 1 diabetes, a closed-loop insulin delivery system. In 2016, the MiniMed 670G became the first FDA-approved continuous blood glucose monitoring system, but at the time the FDA required only patients over the age of 14 to use it. 16. The fourth certified product is the Deep Brain Stimulation (DBS) Clinical System and the ActivaProgramming application. The DBS system is surgically implanted into the patient's brain to provide electrical stimulation to selected areas. The ActivaProgramming application streamlines workflow and provides actionable information for neurologists and neurosurgeons. 17. Dexcom's integrated continuous blood glucose monitoring system (iCGM) Dexcom G6 is the first approved interoperable continuous blood glucose testing system and the only FDA-approved iCGM for other compatible medical devices and electronic interfaces. Integration, such as automatic insulin delivery systems, insulin pumps, blood glucose meters and other diabetes management electronic devices. 18. The FDA approved Insulet Corporation's product OmniPod Dash insulin management system to enter the market. The product includes a wearable insulin pump that can help patients with treatment by controlling the basal rate and dosage. The Dash system also includes a handheld personal diabetes manager with a touch screen that can be connected to the pod via Bluetooth. 19. Eversense, an implantable continuous blood glucose monitoring system from Senseonics, has been approved by the FDA and will be available in the US. The company has already sold its second-generation equipment in Europe, the Middle East and Africa. The third category: cardiovascular 20. PhysioWave, a pulse wave velocity (PWV) cardiovascular analyzer from Stanford University, is FDA-approved. It can be used to monitor blood vessels, pulses and body weight from the heart to the body to determine if a user has a heart. Vascular disease. twenty one. Israel's deep learning startup Zebra Medical Vision received FDA's 510(k) certification, and the company's Coronary Calcium Scoring algorithm can help doctors quantify the extent of coronary calcification in patients with computed tomography. twenty two. PhysIQ's atrial fibrillation detection analysis engine is approved by the FDA for patient care and clinical trials. The engine can be used with other PhysIQ approved products to provide data analysis. twenty three. FibriCheck announced that its application has received FDA 510(k) certification, which uses a smartphone camera and artificial intelligence to monitor arrhythmias. The company expects to bring its products to the US market by 2019. twenty four. The PocketECG CRS, a mobile cardiac rehabilitation system from MediLynx, a subsidiary of Medicalgorithmics, received FDA 510(k) certification. Based on PocketECG technology, the product can remotely monitor arrhythmia patients in 30 days. The PocketECG CRS also includes a built-in accelerometer and a touch screen that transmits data to the online monitoring platform and the doctor portal. The fourth major category: neurology 25. Empracea's Embrace, a wearable device for detecting epilepsy, received FDA's 510(k) certification in January. This product has been used in clinical trials by the pharmaceutical company Sunovion, but users must obtain a prescription from a neurologist to use the device's epilepsy detection function. 26. MemoryMD's two products, NeuroEEG and NeuroCap, are FDA-approved. NeuroEEG is a wireless amplifier worn on the arm that transmits EEG signals to computers and cloud databases via Bluetooth. The NeuroCap is a 19-channel disposable EEG headset that can be used with both products. 27. Rapid CTA, a product of cerebrovascular imaging company iSchemaView, is a 3D imaging platform for computed tomography (CTA). CTA scans can help clinicians visualize the patient's cerebral arteries. 28. In June 2018, MindMaze received the FDA's certification of MindMotion Go, a home neurological rehabilitation platform for moderate to moderate injury. Using Microsoft Kinect-based motion capture technology, the product allows patients to perform a range of activities in a 3D environment to promote recovery. 29. The wearable ear nerve stimulation device from DyAnsys, a medical device company, has been approved by the FDA to treat opioid withdrawal symptoms. 30. SPR Therapeutics has launched the Sprint Peripheral Neurostimulation (PNS) system, a wearable pain management device that supports rechargeable batteries and controllers with Bluetooth. 31. PEAR Therapeutics' product, ReSET, is the first FDA-approved digital prescription product to be used in the adjuvant treatment of drug abuse disorders such as stimulants, marijuana, cocaine or alcohol to help patients regain control of drug addiction. The fifth major category: telemedicine 32. ToSense's CoVa monitoring system, a remote patient monitoring necklace, received a second FDA approval this year, allowing the company to update its software to add new features to its original product, measuring stroke volume and output. In addition, the device is allowed to connect to a mobile application that allows medical professionals to view measurement data remotely. 33. Interconnect Health Company Kinsa has launched a wireless device called Kinsa QuickCare. The QuickCare thermometer reads the temperature in 8 seconds and retains most of the software features of the previous product Smart Stick. 34-37. Smart Inhaler Inc. Adherium has received FDA 510(k) certification for its SmartTouch for Symbicort, an inhaler monitoring device for AstraZeneca's Symbicort aerosol inhalers. The device can be mounted to a patient's inhaler to monitor and improve medication compliance and to help clinicians identify when and how the patient misuses the inhaler. A few months later, Adherium's other products for the ProAir HFA, Ventolin HFA and Flovent HFA inhalers were also approved by the FDA for over-the-counter approval. 38. The BioStamp nPoint system from sensor manufacturer MC10 was first certified by the FDA for 510(k). The system consists of reusable sensor patches that monitor users 24 hours a day. The sensor can record various vital signs, such as exercise and heart rate, and display the relevant data on the mobile phone also provided by MC10. Hotel Safe Box,Password Hotel Safe Box,Hotel Electronic Safe Box,Safe Box Hotel Room Hebei Yingbo Safe Boxes Co.,Ltd , https://www.yingbosafebox.com