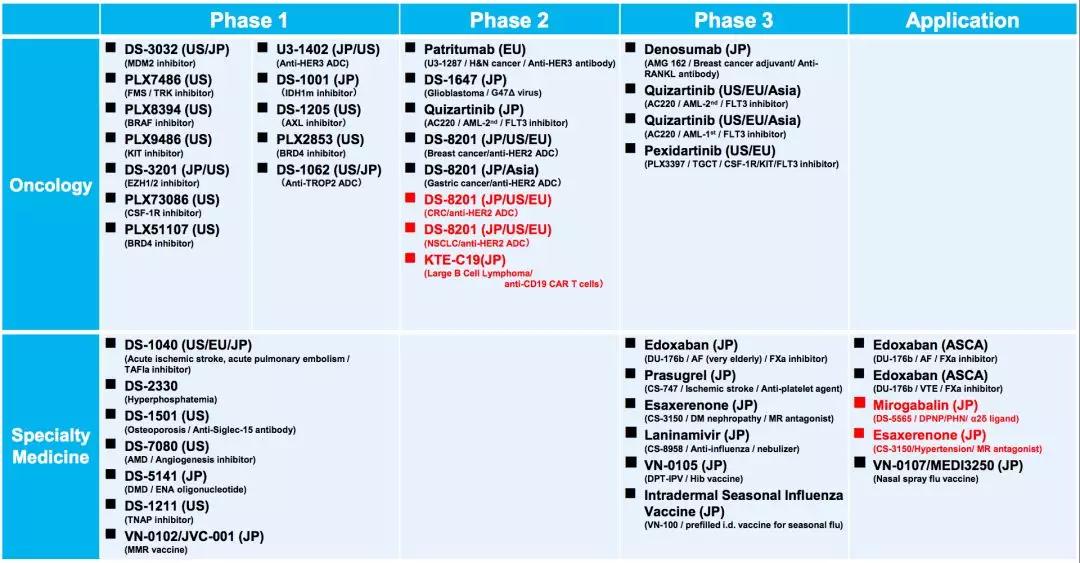
The risk of death is reduced by 24%! Leukemia new drug announced phase 3 results June 19, 2018 Source: WuXi PharmaTech Recently, Daiichi Sankyo announced at the 23rd Congress of the European Society of Hematology that the Quantum-R, a key phase 3 clinical trial led by the University of Texas MD Anderson Cancer Center, showed that quizartinib has extended FMS-like internal tandem repeats. (FLT3-ITD) Gene mutations in the fatal form of acute myeloid leukemia (AML) patients with overall survival. AML is a malignant blood and bone marrow cancer. Uncontrolled dysplastic leukocytes accumulate and accumulate in an uncontrolled manner, resulting in the formation of normal blood cells. According to a 2005-2011 survey, AML patients have a 5-year survival rate of only 26%, the lowest of all types of leukemia. Mutations in the FLT3 gene are the most common genetic variation in AML patients, while FLT3-ITD mutations are the most common mutation in the FLT3 gene, and approximately one in four AML patients carry this mutation. Patients with mutations in the FLT3-ITD gene have a worse prognosis, and their cancer recurrence is increased compared with patients who do not have this mutation, and the risk of death after relapse increases. And even with hematopoietic stem cell transplantation (HSCT), the risk of cancer recurrence after treatment will still increase. These patients urgently need effective treatment to alleviate the disease and prolong life. Quizartinib is an oral specific FLT3 inhibitor developed by the First Triad Corporation. FLT3 is a tyrosine kinase that continues to inhibit its activity leading to apoptosis in tumor cells. However, past tyrosine kinase inhibitors or FLT3 inhibitors often have a variety of side effects due to insufficient potency or specificity, which result in insufficient dose to provide sustained inhibition of FLT3 activity. Therefore, previous FLT3 inhibitors cannot treat AML as a monotherapy. Quizartinib is significantly potent compared to previous generation FLT3 inhibitors and is sufficiently specific that it completely inhibits FLT3 activity at doses tolerated by patients. The published QUANTUM-R is a key global, open-label, randomized phase 3 study involving 367 patients with AML with FLT3-ITD mutations who were treated with standard first-line AML (with or without HSCT). Refractory or relapse (remission duration less than 6 months). They were randomized to receive oral monotherapy quizartinib (60 mg, 30 mg introduction) or salvage chemotherapy in a 2:1 ratio. The primary end point of the study was whether quizartinib prolonged overall survival compared with salvage chemotherapy. The study showed that patients who received quizartinib had a 24% lower risk of death (HR = 0.76, P = 0.0177, 95% CI 0.58-0.98) compared with patients receiving salvage chemotherapy. The median overall survival was 6.2 months in patients receiving salvage chemotherapy (both bilateral 95% CI 5.3-7.2), compared with 4.7 months in patients receiving quizartinib (bilateral 95% CI 4.0-5.5). Patients who received quizartinib had a survival rate of 27% at one year and 20% for patients receiving salvage chemotherapy. â–²Daiichi Sankyo's research product line (Source: Daiichi Sankyo official website) "The FLT3-ITD mutant AML represents a highly unmet need because patients with this form of invasive disease have a poor overall prognosis compared to patients without this mutation, and their remission rate using existing available therapies is low. The risk of recurrence is high and the overall survival is short," said Dr. Jorge E. Cortes, vice president of the Department of Leukemia, Department of Cancer Medicine, MD Anderson Cancer Center, University of Texas, USA. "In the relapsed/refractory AML of the FLT3-ITD mutation, these findings represent The first clinical data reported that a single drug significantly improved overall survival, suggesting that quizartinib has the potential to prolong the lifespan of these patients. In addition, in this study, more patients in the quizartinib group received stem cell transplantation compared with the chemotherapy group." "The results of this study are consistent with previous Phase 2 studies and demonstrate the value of targeting FLT3-ITD-driven mutations. We are satisfied with these data and they will be the basis for submission of regulatory advice to the health sector. If approved, quizartinib It is possible to redefine the treatment options for patients with relapsed/refractory AML with FLT3-ITD mutations," said Dr. Antoine Yver, executive vice president and global head of the oncology development department at the first three companies. "These results are based on our Understanding the types of AML that are difficult to treat. We will continue to explore the potential role of quizartinib in combination with chemotherapy and other new mechanisms to further advance the relapse/refractory of FLT3-ITD mutations and the treatment of newly diagnosed AML patients." Reference materials: [1] Phase III study shows quizartinib prolongs overall survival for patients with deadly type of relapsed or refractory AML [2] Phase 3 QuANTUM-R Study Demonstrates Daiichi Sankyo's Quizartinib Significantly Prolongs Overall Survival as Single Agent Compared to Chemotherapy in Patients with Relapsed/Refractory AML with FLT3-ITD Mutations [3] Significantly prolongs patient life! Leukemia New Drug Program applies for global listing Masks Disposable medical masks are sterilized and meltblown three-layer protective surgical masks for male and female adults, strictly inspected, disinfected and sterilized. Dust-free workshop, specialized equipment, source manufacturer. Carefully packaged, safe and hygienic. Dust-free workshop, quality control. Professional equipment, source manufacturer. Automated production, quality assurance. Real material, serious and responsible. China Ffp2 Face Mask,Ffp2 KN95 Mask manufacturers, welcome Ffp2 Medical Facemask,Ffp2 Medical Respirator purchasers from worldwide to visit our site. Ffp2 Face Mask,Ffp2 Kn95 Mask,Ffp2 Medical Facemask,Ffp2 Medical Respirator KUTA TECHNOLOGY INDUSTRY CO.,LIMITED , https://www.kutasureblue.com